Abstract
INTRODUCTION
Treatment outcomes in AL amyloidosis are dependent on the hematological response to chemotherapy translating into organ responses. Cardiac biomarker, N-terminal brain pro-natriuretic peptide (NT-proBNP), is the main determinant of cardiac response in AL amyloidosis. Strongly supported by the amyloidosis community, the FDA is considering use of NT-proBNP as the primary end point for clinical trials. A number of questions on the exact details on NT-proBNP in AL remain unanswered - rate of decrease over time, timing of NT-proBNP measurement (6 months or 12 months) and values to use as a baseline. We report the use of serial NT-proBNP measurements for cardiac response assessment at 6 months and 12 months addressing some of these questions.
PATIENTS AND METHODS
All patients (n=650) recruited in the ALChemy study (a prospective observational study of all patients with AL amyloidosis undergoing chemotherapy) at the UK National Amyloidosis Centre from Sept 2009 to Jan 2014 with a minimum follow of 12 months and available NT-proBNP at 0, 6 and 12 months were included in this study. Organ involvement and hematologic/amyloidotic organ responses were assessed according to 2010 amyloidosis consensus criteria. The primary outcome measure was cardiac response as defined by NT-proBNP (Palladini et al JCO 2012). Correlation with 6 minute walk test was done where available.
RESULTS
A total of 343 patients were identified. The median age was 64 yrs. Organ involvement was: cardiac - 72%, renal - 73% and liver - 12% (median - 2 organs). The median creatinine was 90 _mol/L, median dFLC was 150 mg/L (range 10-15898 mg/L). The median NT-proBNP was 966 ng/L (range 33-30872 ng/L). The Mayo disease stage was: stage 1 - 26%, stage 2 - 47% and stage 3 Ð 27%. Serial six minute walk test results were available in 71 patients. Treatment was: thalidomide based regimes (mainly CTD) 56%, CyBorD Ð 30%, Melphalan-Dexamethasone - 5% and SCT 1%.
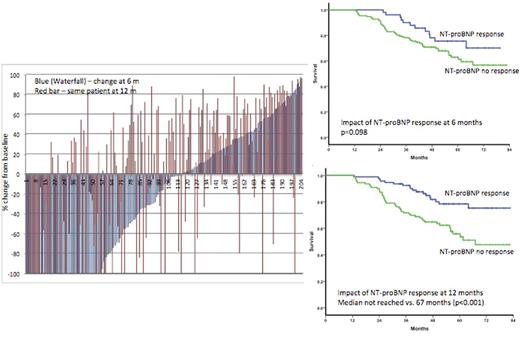
A total of 204 patients had baseline NT-proBNP >650 ng/L (the threshold defined for NT-proBNP to be assessable for cardiac response) and were included in response analysis. Partial hematological response (or better) was seen in 92%, ³ VGPR in 66% and 22 (8%) were non-responders. The median decrease in dFLC was 85% over baseline at six months. The median NT-proBNP at baseline (for the response assessable group n=204) was 2669 ng/L. At six months, the median NT-proBNP had increased significantly to a median 3258ng/L (p<0.0001). At 12 months, there was a significant decrease in the NT-proBNP to a median of 2097 pMol/L (p=0.014). There was a discordance in NT-proBNP response at 6 and 12 months in 42 (44%) of patients (Figure 1). At six months, 52 (25%) patients had achieved NT-proBNP response and at 12 months 94 patients (46%) achieved an NT-proBNP response. When NT-proBNP at the 6 month time point was used as a baseline for response assessment, at 12 months, 106 (52%) patients met the criteria for a cardiac response. There was no significant difference in the median six minute walk test at baseline, 6 m and 12 months was 390m, 370m and 400 m. The six minute walk distance improved by greater than 10% over baseline at 6 and 12 months in 12% and 20% patients, worsened by more than 10% in 32% and 27% patients with change of less than 10% in the remainder.
The median survival for this cohort has not been reached at 5 years with 60% survival at 7 years. Contrary to published data, NT-proBNP response at six months had a non-significant impact on survival whilst the NT-proBNP response at 12 months significantly impacted survival (median not reached for responders vs. 67 months for the non-responders; p<0.0001).
CONCLUSIONS
This study shows the median NT-proBNP increased at six months over the baseline. There was a marked discrepancy in NT-proBNP at 6 months and 12 months Ð with a clear mis-classification of nearly half of all eventual cardiac responders. Consequently, the NTproBNP measurements at six months did not have a significant impact on survival whilst there was a marked impact at 12 months. NT-proBNP, as a primary end point, measure too early has potential for giving false negative results as a trial end point. This study highlights the critical importance of the timing of NT-proBNP measurements in response assessment for AL amyloidosis for clinical trials to avoid false negative results.
Wechalekar:Janssen: Honoraria; Celgene: Honoraria; Glaxo Smith Kline: Honoraria; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal