Abstract
Introduction: There is limited data on the best treatment strategy for patients who relapse after upfront arsenic trioxide (ATO) based therapy for acute promyelocytic leukemia (APL). We had recently reported that there was significant micro-environment mediated drug resistance (EM-DR) to ATO which we noted was predominantly mediated by upregulation of the NF-ⱪB pathway. We also noted that this upregulation was more prominent in relapsed APL and that EMDR to ATO could be overcome by the use of proteasome inhibitors (Leukemia 2016 - In Press). Pre-clinical mouse model and preliminary clinical data validated these observations. There has also been a recent concern of mutations in the oncogenic PML-RARA gene following treatment with ATO, with reports suggesting a third of relapsed APL patients having such mutations and that the clinical outcomes in this subset with conventional therapy is poor [NEJM. 2014;370(19): 1864-6].
Methods: In this prospective, open-label, non-randomized, phase 2, single center study, we enrolled APL patients in first hematological relapse. Inclusion criteria included presence of t(15; 17) and exposure to ATO as part of up front therapy. Enrolled patients received conventional therapy for relapse, included ATO and ATRA in induction, consolidation. Anthracycline (mitoxantrone) was used only in induction. Additionally, patients received two doses of bortezomib (1.4mg / m2 / dose) in induction and consolidation on days 2 and 5 and one dose in maintenance (10 days/month x 6 months) on day 2. Primary end point was safety (graded according to the NCI Common Terminology Criteria for Adverse Events version 4.0) and the key secondary end points were proportion in complete molecular remission at the end of induction and event free survival (EFS). The study is ongoing and continues to recruit patients. This trial is registered with ClinicalTrials.gov, number NCT01950611.
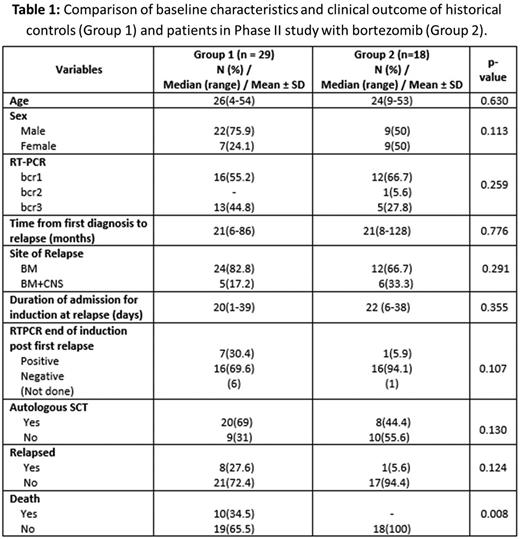
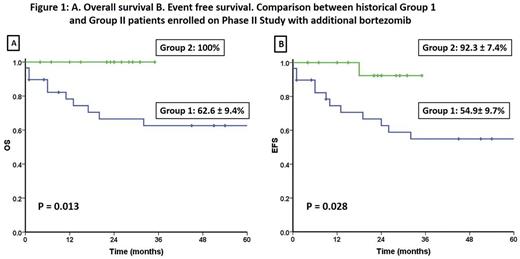
Results: Between Sep 2013 and Mar 2016, 18 patients met eligibility criteria and were enrolled in this study. All 18 patients achieved CHR at a median of 45 days (range: 42-63). Seventeen patients (94.4%) were RT-PCR negative at the end of induction. The median time complete molecular remission was 35 days (range: 21-56 days). None of the patients had any major bleeding or thrombotic events in induction. With the exception of one patient who had Grade IV neurotoxicity which required treatment and recovered after temporarily discontinuing treatment during induction none of the other patients had any Grade III/IV non-hematological toxicity. Other non-hematological events were ≤ Grade II, usually transient and judged to be unrelated to the interventional agent. These included mild headache in 8 (33.3%) and one case (5.5%) each with diarrhea, mild rash, redness of eyes, oral ulcers, vertigo and paresthesia. Auto-SCT was offered to all patients in molecular remission post consolidation. Eight patients (44.4%) underwent an autologous SCT post consolidation while the rest opted to receive maintenance therapy. One patient who opted for maintenance therapy relapsed 6 months after completing maintenance. At an actuarial median follow up of 24 months (range: 4-35) the 3 year KM estimate of EFS was 92.3±7.4% and the cohort had 100% survival at last follow up. This clinical profile and outcome data was compared with a historical cohort that was treated with the same protocol with the exception of bortezomib and the comparative data is summarized in table 1 and figures 1A and 1B.
Conclusion: Addition of proteasome inhibitors with ATO is well tolerated. In combination with conventional agents, proteasome inhibitors can potentially improve the clinical outcome of patients with relapsed APL treated with upfront ATO. Our preliminary data also suggests that this combination can obviate the need for an auto-SCT in this setting.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal