Abstract
Background
The combination of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) has showed advantages over standard ATRA and chemotherapy (CHT) in the treatment of low- and intermediate-risk acute promeylocytic leukemia (APL). However, the first-line therapy of high-risk APL is controversial, particularly concerning ATO and CHT. A phase 4, prospective, randomized, open-label, multicenter trial was conducted in China to see if CHT could be replaced by ATO in patients with low- and intermediate-risk APL, and if CHT could be minimized by ATO in patients with high-risk disease.
Methods
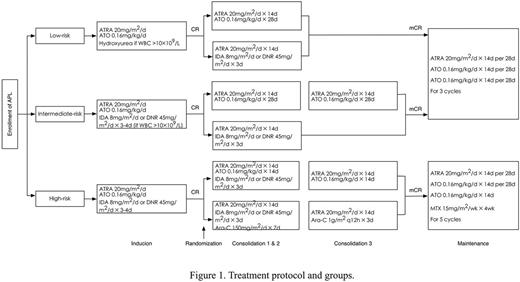
Patients all received ATRA and ATO for induction therapy, combined with anthracycline for patients at high risk and those at intermediate risk but once the white blood cell count was over 10¡Á109/L during induction. In consolidation phase, patients of low-risk APL randomly received either 2 cycles of ATRA-ATO (experimental group) or 2 cycles of ATRA-CHT (reference group) treatment. Patients of intermediate-risk disease randomly received either 3 cycles of ATRA-ATO (experimental group) or 2 cycles of ATRA-CHT (reference group), whereas patients of high-risk disease were randomized to receive either 2 cycles of ATRA-ATO-anthracycline and 1 cycle of ATRA-ATO (experimental group) treatment or 2 cycles of ATRA-anthracycline-cytarabine and 1 cycle of ATRA-mid dose cytarabine (reference group). Once they achieved molecular remission, patients of low- and intermediate-risk continued with 3 cycles of maintenance therapy including ATRA and ATO, while those of high-risk received 5 cycles of ATRA, ATO and methotrexate (MTX) treatment (Figure 1).
Results
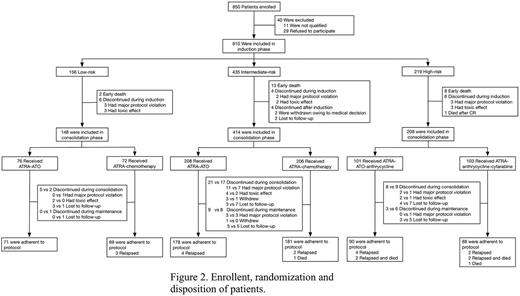
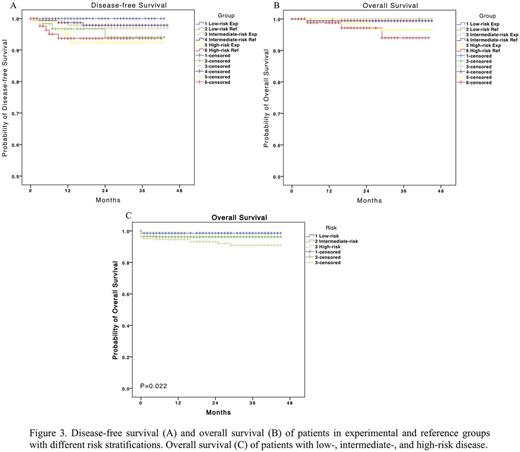
A total of 794 eligible patients have been enrolled in the trial since Jul 2012 and started the assigned treatment with informed consent (Figure 2). Complete remission was achieved in 771 of 794 (97.1%) patients. Early death occurred in 23 patients (2.9%). The median follow-up was 23 months (range, 0 to 45). A total of 19 patients relapsed. In patients with low-risk APL, the 3-year estimated disease-free survival rate was 100% and 94.1% in the experimental group and the reference group, respectively (P=0.088). In patients with intermediate-risk disease, it was 96.9% vs 97.9% (P=0.651). And in patients with high-risk disease, it was 92.4% vs 93.7% (P=0.897; Figure 3A). Overall survival (Figure 3B) and event-free survival were also of no significant difference between experimental and reference groups with each risk stratification. And the 3-year overall survival for patients with low-, intermediate-, and high-risk APL was 98.6%, 96.2%, and 90.8%, respectively (P=0.022; Figure 3C).
Conclusion
ATRA and ATO combination with or without CHT as the induction therapy achieved as high remission rate as in Shanghai Regimen, meanwhile the application of CHT to those with hyperleukocytosis during induction therapy might benefit them with reduced occurrence of early death. ATRA-ATO is not inferior to ATRA-CHT in patients with low-risk APL. ATO could replace CHT in post-induction phase in patients with intermediate-risk APL. And CHT could be minimized by adding ATO as a substitute for cytarabine in patients with high-risk disease. (Funded by the National High-tech Research and Development Program [863 Program] of China 2012AA02A505 and others; ClinicalTrials.gov identifier: NCT01987297.)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal