Abstract
Background: Chemoimmunotherapy regimens remain the preferred first-line treatment for most of patients with CLL/SLL. Recent studies have demonstrated the efficacy of maintenance regimens by improving progression-free survival (PFS). We studied safety and efficacy of a treatment protocol that included induction and maintenance components using vorinostat, a histone deacetylase inhibitor, in combination with FCR for induction and with rituximab for the maintenance.
Methods: Previously untreated CLL/SLL patients with indication for treatment were eligible. Primary objective of the Phase I part was the maximum tolerated dose (MTD) of vorinostat that could be combined with FCR and PFS was the primary endpoint for phase II. In the induction phase, FCR (fludarabine 25 mg/m2 days 1-3, cyclophosphamide 250 mg/m2 days 1-3 and rituximab 375 mg/m2 on day 1 of cycle 1 and 500 mg/m2 on day 1 of subsequent cycles) was given every 28 days for 6 cycles. In addition to FCR, patients received vorinostat orally on days 1-5 and days 8-12 of each treatment cycle. In the dose finding phase, vorinostat was given at 3 dose levels: 200 mg, 300 mg and 400 mg PO daily. There was no dose limiting toxicity and 400 mg was chosen as the MTD for phase II. Patients who received at least 4 cycles of induction were eligible to receive maintenance beginning within 3 months of the last cycle of FCR. In the maintenance phase, given every 3 months for 8 cycles, rituximab was given at dose of 500 mg/m2 on day 1 and vorinostat was given at 400 mg PO daily on days 1-14 of each cycle.
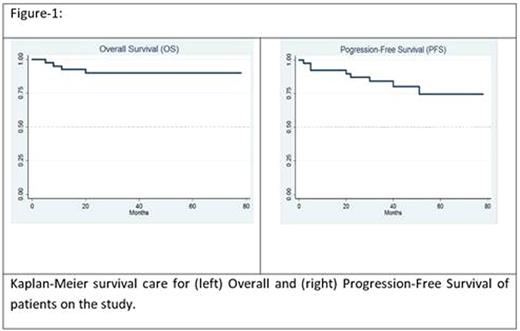
Results: 40 patients (CLL 32, SLL 8) were treated. Median age was 58 years (36-72). Median time from diagnosis to treatment was 27 months (0 -102). Rai stage at the time of treatment was I/II in 23 (60%) and III/IV in 17 (40%) patients. Cytogenetic (CG) abnormalities included del17p in 2 (5%), del11q in 2 (5%), trisomy 12 in 4 (10%), del13q in 3 (7.5%), normal CG in 14 (35%) and other abnormalities in 6 (15%) patients. Median number of induction cycles was 6 (1-6) and 33 patients (83%) patients finished at least 4 cycles of induction and were eligible for maintenance. Reasons for delivery of less than 4 induction cycles were patient preference (2), GI toxicity (1), thrombocytopenia (1), pulmonary emboli (1), progressive disease (1) and sepsis (1). Twenty-six patients (65%) received at least 1 cycle of maintenance treatment (median 8; range, 1-8) and 15 patients (40%) finished all 8 planned maintenance cycles. Eleven patients started maintenance treatment but did not receive all 8 maintenance courses due to neutropenia (3), patient preference (3), infections (1), insurance issues (1) or other reasons (3). Six patients (15%) were eligible for maintenance but did not receive it because of hematologic toxicities (3), infection (1) or patient preference (2). Overall response rate after induction was 91% with CR in 26 (74%), PR in 4 (11%), nPR in 2 (6%) and stable disease (SD) in 1 (3%). One patient (3%) had progressive disease during the induction and 1 (3%) was not evaluable. Twenty-one of 23 (88%) patients who achieved CR after induction, were tested for minimal residual disease (MRD) by flow cytometry and cytogenetics and 21 (91%) had negative MRD status. One of 2 CR patients who was MRD positive after induction converted to MRD negative after maintenance. After median follow-up of 42 months, 5-year estimate of overall survival was 90% (95% CI 75%-96%) and 5-year estimate of PFS was 72% (95% CI 51%-85%). None of the patients who achieved CR after induction (n=26; 74%) had disease progression after median 46 months of follow-up while 3 of 6 patients with PR or nPR had disease progression which occurred median of 40 months (29-51). Most common grade 3-4 toxicities were hematologic (36%), electrolyte abnormalities (9%), gastrointestinal (8%) and cardiovascular (6%). 4 patients (10%) died during the follow-up because of disease progression (1), sepsis (1), fungal infection (1) and suicide (1). Median time to death was 9.5 months (5 -20).
Conclusion: Combination of vorinostat with FCR for induction and with rituximab for maintenance was feasible and effective with a high ORR and long OS and PFS with no relapses observed among patients who achieved CR after induction. Cytopenias and infections were main reasons for treatment discontinuation especially during the maintenance phase. Combination of novel agents with conventional induction and maintenance regimens is a promising strategy for treatment of CLL/SLL.
Shadman:Gilead: Honoraria, Research Funding; Emergent: Research Funding; Acerta: Research Funding; Pharmacyclics: Honoraria, Research Funding. Gopal:Paid Consultancy- Gilead, Janssen, Seattle Genetics, Spectrum, Research funding- Gilead, Janssen, Pfizer, BMS, Merck, Teva, Takeda, Spectrum, Seattle Genetics: Consultancy, Honoraria, Research Funding. Press:Roche / Genentech: Consultancy, Research Funding. Maloney:Juno Therapeutics: Research Funding; Genentech/Roche: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal