Abstract
Introduction:
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib (IB) is currently approved for the treatment of patients with chronic lymphocytic leukemia and small lymphocytic lymphoma (CLL/SLL), including patients with del17p disease. Treatment with IB is effective and generally well-tolerated. However, a proportion of patients experience intolerable adverse events (AEs) requiring IB discontinuation. Non-infectious toxicities are generally considered to be due to kinase inhibition and it is uncertain if the AE is directly attributable to inhibition of BTK (on-target effect) or to inhibition of an alternative target of ibrutinib (off-target effect). To indirectly examine this, we reviewed toxicity and tolerability data for patients who discontinued IB due to significant AEs and subsequently received an alternative BTK inhibitor.
Methods:
Patients with previously treated CLL/SLL who were intolerant to IB due to non-infectious IB-attributable AEs and subsequently received a different BTK inhibitor were included in this analysis. Patients with infectious AEs were excluded. AEs arising during IB therapy that were attributable to IB and resulted in discontinuation based on both patient and investigator preference were detailed and graded according to CTCAE v.4.03. These AEs were assessed for resolution or increasing severity after discontinuing IB both before and during subsequent BTK inhibitor treatment.
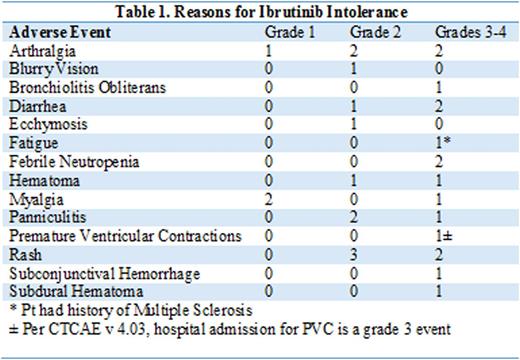
Results:
Twenty-one patients were included in the analysis and the median age was 61-years (range 50-76). Patients had received a median of 4 prior therapies (range 2-11). Fifty-eight percent of the patients had Rai stage 3-4 disease, 76% were IGHV un-mutated, and 57% had del17p. Prevalent co-morbidities by organ system prior to start of acalabrutinib included blood and lymphatic system disorders (48%), infectious issues (52%), cardiac disorders (38%), gastrointestinal disorders (43%) and musculoskeletal disorders (33%). Median time on IB was 13 months (range 1.6-62). Reasons for IB discontinuation are detailed in Table 1. With regards to more severe (grade 3 or 4) IB-attributable AEs, 2 patients experienced grade 4 AEs resulting in discontinuation of IB including neutropenia and subdural hematoma. Both these patients had resolution of their AEs prior to starting subsequent treatment. Among the 14 patients discontinuing IB for grade 3 AEs, 1 had ongoing grade 3 diarrhea, 1 had grade 3 arthralgia, and 2 had grade 2 AEs of diarrhea and fatigue at the time of starting subsequent treatment. Of the 21 included patients, 10 had ongoing IB-attributable grade 1-3 AEs at the time of starting a different BTK inhibitor. Three of these patients experienced resolution of one or more IB-attributable AEs (grade 3 diarrhea, and grade 2 AEs including panniculitis, arthralgia and diarrhea) within 28 days of starting therapy, 6 patients had stable grade 1-2 AEs (grade 1 rash in 2 patients, grade 2 diarrhea, grade 1 arthralgia, grade 1 abdominal pain, grade 2 fatigue) at last follow up while taking alternate BTK inhibitor, and 1 patient with an ongoing grade 2 AE at time of treatment initiation developed CNS involvement with their malignancy and was taken off therapy prior to assessment for resolution of the AE. The remaining 11 patients had either complete resolution or improvement of the AE to grade 1 prior to starting the treatment. No recurrence or worsening of IB-attributable AEs were observed during a median follow-up of 13-months on alternative treatment except for 1 patient with hemophilia A, who developed grade 3 hematoma while on IB therapy and experienced recurrent hematoma after 5 days of treatment with a different BTK inhibitor, at which time the drug was discontinued.
Conclusions:
For patients who discontinued IB due to non-infectious IB-attributable AEs, subsequent treatment with an alternative BTK inhibitor does not appear to aggravate or precipitate IB-attributable AEs that had previously resulted in discontinuation. This supports attribution of these AEs to off-target effects of ibrutinib and not inhibition of BTK. The use of an alternate BTK inhibitor may be a viable therapeutic strategy for patients who discontinued IB due to intolerable AEs without experiencing clinical progression.
Jones:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, LLC, an AbbVie Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Woyach:Karyopharm: Research Funding; Morphosys: Research Funding; Acerta: Research Funding. Awan:Innate Pharma: Research Funding; Novartis Oncology: Consultancy; Pharmacyclics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal