Abstract
Background
Prognostic factors correlate with clinical outcomes independent of treatment. Many prognostic factors have been identified in CLL. More effective treatments, like B cell receptor (BCR) signaling pathway inhibitors, have the potential to nullify the prognostic impact of some markers. Time-to-first treatment is an endpoint unaffected by choice of treatment. CpG-stimulated metaphase karyotype can reliably and reproducibly identify cytogenetic abnormalities in CLL that may not be seen with standard non-stimulated karyotype or by FISH. Complex cytogenetics, defined as 3 or more chromosome abnormalities identified in 2 or more metaphases was the highest-risk feature for shorter progression-free and overall survival in patients receiving ibrutinib for relapsed/refractory CLL. Complex karyotype is not uncommon among relapsed/refractory CLL cases, particularly those who previously received genotoxic chemotherapy. The frequency and impact of karyotype abnormalities have not previously been reported for treatment-naïve CLL.
Methods
We evaluated treatment-naïve patients with CLL who had their initial evaluation, which included prognostic factor assessment, at MDACC between July 2013 and June 2016. CpG-stimulated metaphase karyotype of CLL cells from blood or bone marrow was performed by culture of mononuclear cells for 72hrs in media containing CpG-685 (20ug/ml), phorbol 12-myristate 13-acetate (PMA; 0.04ug/ml) and Pokeweed mitogen (PWM; 0.1ug/ml). Banding and analyses were by standard laboratory procedures. Twenty metaphases were analyzed per culture and patients were categorized as having diploid karyotype, a single clonal chromosome abnormality present in more than 1 metaphase, two clonal chromosome abnormities present in more than 1 metaphase, or 3 or more chromosome abnormalities identified in more than 1 metaphase (complex).
Results
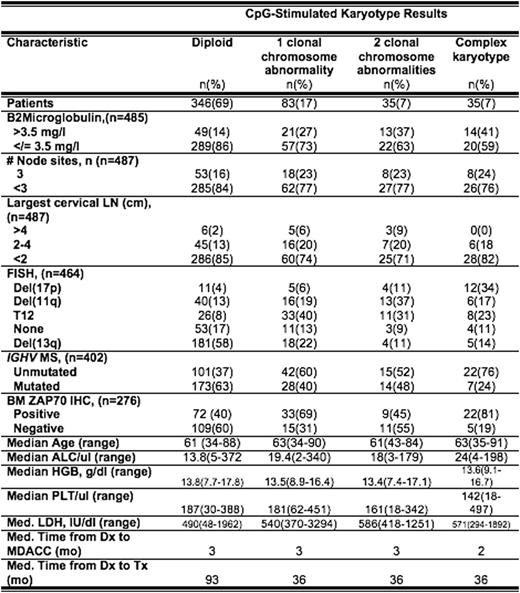
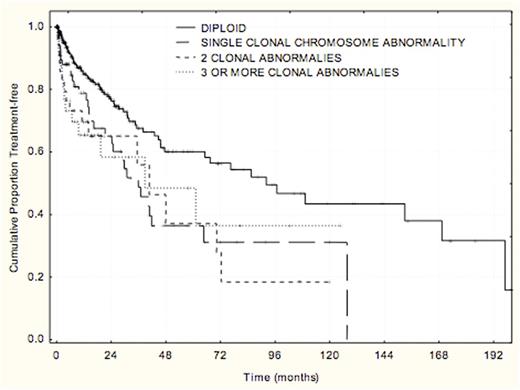
CpG-stimulated karyotype was obtained in 500 treatment-naïve patients with CLL. The frequency and distribution of chromosome abnormalities with other prognostic factors and time-to-first treatment were analyzed (Table). The majority (69%) of patients had diploid cytogenetics. Higher-risk prognostic features such as del(17), del(11q), unmutated IGHV and ZAP70 expression were associated with presence of complex karyotype abnormalities. Shorter time-to-first treatment from diagnosis was associated with 1, 2, and 3 or more clonal chromosome abnormalities compared to diploid karyotype (p=.0005) (Figure).
A previous multivariable model identified the following independent characteristics correlated with time-to-first treatment: size of the largest cervical lymph node, number of nodal sites involved, FISH cytogenetics, LDH, and IGHV mutation status (Wierda et al. JCO 29:4088, 2011). We are working to integrate stimulated cytogenetics results into this multivariable model for time-to-first treatment.
Conclusions
Clonal chromosome abnormalities were identified in nearly 30% of previously untreated patients with CLL and were associated with shorter time-to-first treatment. Analyses are ongoing to determine optimal integration into a multivariable model for time-to-first treatment.
Jain:Celgene: Research Funding; Novimmune: Consultancy, Honoraria; Genentech: Research Funding; Seattle Genetics: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; Infinity: Research Funding; Novartis: Consultancy, Honoraria; Abbvie: Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; BMS: Research Funding. Thompson:Pharmacyclics: Consultancy, Honoraria. Burger:Gilead: Research Funding; Roche: Other: Travel, Accommodations, Expenses; Janssen: Consultancy, Other: Travel, Accommodations, Expenses; Portola: Consultancy; Pharmacyclics, LLC, an AbbVie Company: Research Funding. O'Brien:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria. Wierda:Genentech: Research Funding; Gilead: Research Funding; Acerta: Research Funding; Abbvie: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal