Abstract
Background
Isocitrate dehydrogenase 1 and 2 mutations (IDH1/2MT) occur in up to 20% of MDS, MDS/MPN overlap and (secondary) AML, 80% of secondary glioma and 20% of cholangiocarcinoma patients. IDH1/2MT confer an improved prognosis in glioma and cholangiocarcinoma patients, but not AML patients, compared with IDH1/2 wild-type (IDH1/2WT)counterparts (Fig 1). IDH1/2MT enzymes produce D-2-hydroxyglutarate (D-2HG)at the expense of NADPH, an important cellular antioxidant. IDH1/2MT are ancestral in ~30% of AML/MDS cases, rendering them attractive therapeutic targets because all cancer cells carry IDH1/2MT and this limits the chance of therapy resistance. Inhibitors of IDH1/2MT were recently developed and are currently in clinical trials. IDH1MT inhibitors protect IDH1MT solid tumor cells against irradiation and cisplatin. Thus, IDH1/2MT inhibitors and cytotoxic drugs should not be used together in patients with IDH1/2MT solid tumors. It remains unknown whether IDH1/2MT inhibitors can be safely used as adjuvants to cytotoxic drugs in patients with myeloid malignancies.
Methods
Primary IDH1/2MT and IDH1/2WT AML and MDS cells (n=20) were cultured and treated with D-2HG, the IDH1/2MT inhibitors, the antioxidant N-acetyl cysteine (NAC) and/or irradiation, cisplatin, 5-azacytidine, cytarabine, daunorubicin or decitabine. We determined cell death, ROS levels and DNA double strand break numbers. In addition, maximal cellular IDH1/2 and glucose-6-phosphate dehydrogenase (G6PD) activity was determined.
Results
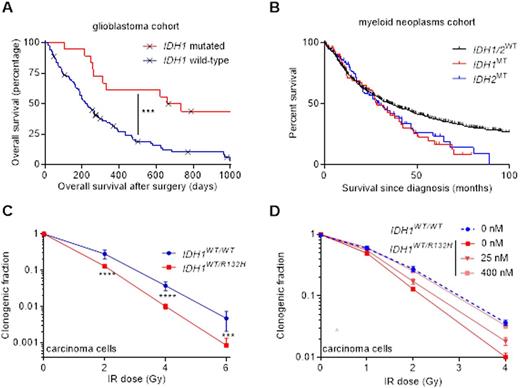
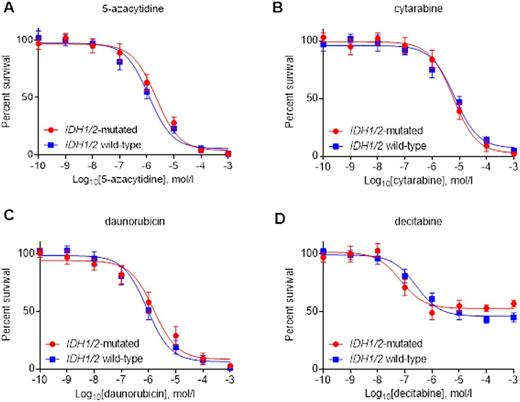
After treatment with irradiation, cisplatin, 5-azacytidine, cytarabine, daunorubicin or decitabine, we did not observe more reactive oxygen species (ROS), DNA double strand breaks or cell death in IDH1/2MT primary AML/MDS cells versus IDH1/2WT primary AML/MDS cells (Fig 2). Accordingly, IDH1/2MT inhibitors or the ROS scavenger NAC had no effect on these aforementioned outcomes in IDH1/2MT primary AML/MDS cells. These results contrast the situation in glioma and carcinoma cells, where increased ROS levels, DNA double strand breaks and cell death levels were observed in irradiated or cisplatin-treated IDH1/2MT cancer cells, and where IDH1/2MT inhibitors or NAC reverse these effects (Fig 1).
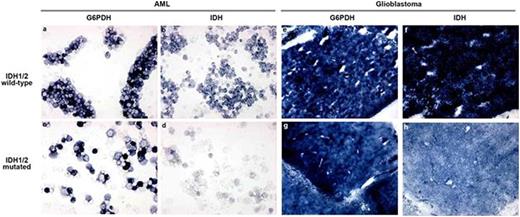
IDH1/2MT were associated with a striking reduction in the IDH1/2-mediated NADPH production capacity in primary AML cells and solid tumor cells. However, IDH1/2 are not important NADPH providers in primary AML/MDS cells and the contribution of G6PD to the total NADPH production pool is ~4 times larger than IDH1/2 (Fig 3). In contrast, IDH1/2 provide 65% of all NADPH in glioma. Administration of D-2HG, the metabolite produced by IDH1/2MT enzymes, decreased IDH1/2-mediated NADPH production capacity in primary AML/MDS cells and solid tumor cells and is a likely cause of the decreased IDH1/2-mediated NADPH production in IDH1/2MT cells.
Discussion
Our results offer an explanation for the relatively longer survival of patients with IDH1/2MT glioma and cholangiocarcinoma and the unchanged survival of patients with IDH1/2MT AML or MDS. A plausible mechanism is the strong production of the antioxidant NAPDH by IDH1/2 in solid tumor, but not AML/MDS cells. These data imply that concomitant use of IDH1/2MT inhibitors and cytotoxic therapy in patients with IDH1/2MT solid tumors may limit therapy efficacy. However, in patients with IDH1/2MT AML or MDS, IDH1/2MT inhibitors can probably be used safely as adjuvants to AML/MDS standard of care 5-azacytidine, cytarabine, daunorubicin and/or decitabine therapies, or whole-body irradiation in the context of bone marrow transplantations. These data are crucial for the rational design of clinical trials with IDH1/2MT inhibitors and complete understanding of their outcomes.
Increased survival of IDH1/2MT patients (A) in a glioblastoma population, (B) but not in a myeloid neoplasm population sequenced and analyzed by our groups. (C) IDH1MT radiosensitize carcinoma cells and (D) IDH1MT inhibitors radioprotect carcinoma cells.
Increased survival of IDH1/2MT patients (A) in a glioblastoma population, (B) but not in a myeloid neoplasm population sequenced and analyzed by our groups. (C) IDH1MT radiosensitize carcinoma cells and (D) IDH1MT inhibitors radioprotect carcinoma cells.
Primary IDH1/2MT AML/MDS cells are not sensitized to (A) 5-azacitidine, (B) cytarabine, (C) daunorubicin or (D) decitabine, compared with primary IDH1/2WT AML/MDS cells.
Primary IDH1/2MT AML/MDS cells are not sensitized to (A) 5-azacitidine, (B) cytarabine, (C) daunorubicin or (D) decitabine, compared with primary IDH1/2WT AML/MDS cells.
G6PD (A, C, E, G) and IDH1 activity (B, D, F, H) staining of IDH1WT (A, B, E, F) and IDH1MT (C, D, G, H) primary AML cells (A-D) and glioblastoma (E-H) cryostat sections. The amount of blue color directly reflects enzyme activity (production of NADPH).
G6PD (A, C, E, G) and IDH1 activity (B, D, F, H) staining of IDH1WT (A, B, E, F) and IDH1MT (C, D, G, H) primary AML cells (A-D) and glioblastoma (E-H) cryostat sections. The amount of blue color directly reflects enzyme activity (production of NADPH).
Carraway:Celgene: Research Funding, Speakers Bureau; Baxalta: Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Maciejewski:Celgene: Consultancy, Honoraria, Speakers Bureau; Alexion Pharmaceuticals Inc: Consultancy, Honoraria, Speakers Bureau; Apellis Pharmaceuticals Inc: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal