Abstract
Background and Object In addition to histone deacetylation, the importance of histone over-acetylation induced oncogene transcription in initiation and progression of myelodysplastic syndrome (MDS) has been proposed recently. Our previous whole-exome sequencing identified a new somatic mutation, ANKRD11, an important factor in histone acetylation regulation. Its roles in MDS pathophysiology need to be clarified.
Methods The next generation target sequencing (Including ANKRD11) was carried out in 320 patients with MDS using the MiSeq Benchtop Sequencer. ANKRD11 mRNA expression in bone marrow of MDS was measured by real-time PCR. Loss and gain of function assay were carried out in myeloid cell lines K562, MEG-01£¬or SKM-1 to observe the influence on cell proliferation and differentiation . The levels of histone acetylation at H3 and H4 were detected by Western blot.
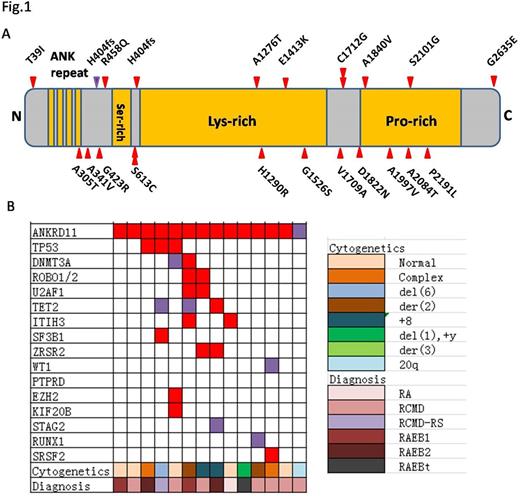
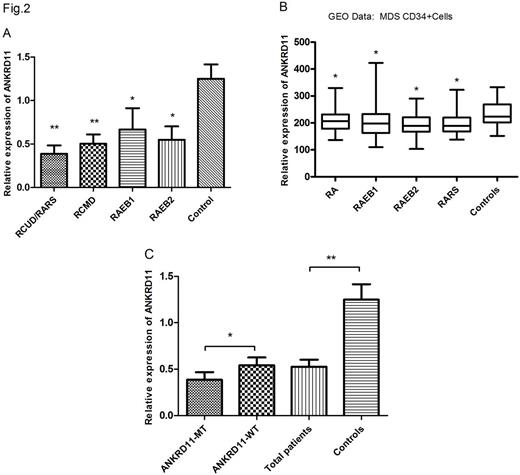
Results Target sequencing in a cohort of 320 MDS patients identified 14 of ANKRD11 mutations (4.38%, Fig.1), which were confirmed by Sanger sequencing. Meanwhile, no ANKRD11 mutations in 100 normal controls were defined. ANKRD11 mutations occurred frequently in exons 10 and 9. The mRNA expression levels of ANKRD11 were significantly decreased in MDS patients, especially in ANKRD11mutant patients (Fig.2). ANKRD11 knockdown in K562 and MEG-1 resulted in growth inhibition, cell cycle arrest and erythroid/megakaryocytic differentiation retardant. In MDS cell line SKM-1, the arrested differentiation was rescued by over-expression of ANKRD11. Consistent with a role for ANKRD11 in histone acetylation, ANKRD11 KD increased acetylation of histones H3 and H4 at H3K14 and H4K5 and resulted in the upregulation of genes involved in differentiation inhibilation (SOX6, P21, et al). Finally, the ANKRD11 KD-mediated influence on cell proliferation and differentiation were reversed by inhibiting histone acetyltransferase activity.
Conclusion Our assay defined that ANKRD11 was a crucial chromatin regulator that suppress histone acetylation and then decrease gene expression during myeloid differentiation, providing a likely explanation for its role in MDS pathogenesis. This study further support histone acetylase inhibitor as a potential treatment in MDS.
ANKRD11mutation distribution (a) and coexist with other mutations (b).
The mRNA expression levels of ANKRD11in our MDS (A, C) subset and GEO data (B).
Changes of histone acetylation in ANKRD11-KD cell line (MEG-01). ANKRD11 KD significantly increased acetylation of histones H3 and H4 at H3K14 and H4K5.
Changes of histone acetylation in ANKRD11-KD cell line (MEG-01). ANKRD11 KD significantly increased acetylation of histones H3 and H4 at H3K14 and H4K5.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal