Abstract
Introduction: The Philadelphia-chromosome negative myeloproliferative neoplasms (MPNs) are clonal hematopoietic stem cell disorders, which include polycythemia vera, essential thrombocythemia and myelofibrosis. These disorders are characterized by activation of the JAK-STAT pathway via somatic mutations in JAK2, MPL, and CALR. However, a number of recurrent somatic mutations outside the JAK-STAT pathway have been described. Many of these mutations, such as ASXL1 and EZH2, are know to confer risk for disease progression. Other mutations, such as TET2 mutations, appear to alter the biology of disease in preclinical and clinical studies. Thus, further investigation of co-occurring mutation events is important to further the understanding of disease biology. Recurrent activating mutations in the RAS signaling pathway have been described in patients with MPNs. We have sought to determine the clinical impact of RAS mutations in patients with MPN, and to assess the pattern of co-mutational events in patients with RAS mutations.
Methods: A targeted 28-gene, amplicon based next-generation sequencing assay was used to sequenced bone marrow aspirate or peripheral blood samples from 211 pts with Philadelphia negative MPN including: ET=61, PV=35, PMF=54, Post ET MF=16, Post PV MF=17, Post MPN AML=20, MPN unclassified=5 and systemic mastocytosis=3. Pts with MDS/MPN overlap syndromes were excluded. NRAS and KRAS mutations were grouped together. FisherÕs exact test and Wilcoxon rank-sum test were used to compare categorical and continuous variables, respectively.
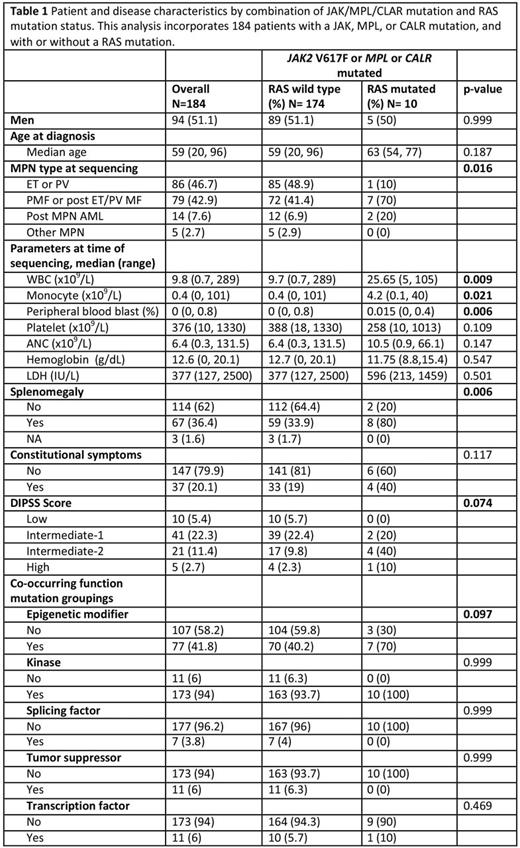
Results: N/KRAS mutations occurred at a frequency of 6 % in this cohort (Fig 1a). The median variant allele frequency (VAF) of RAS mutations was 15.6%. This was lower than the VAF of JAK2 V617F (44.6%) (p<0.001), TET2 (43.0%) (p=0.011) and ASXL1 (29.5%) (p=0.029) suggesting that RAS mutations are sub-clonal relative to other MPN associated mutations. By contrast, mutant RAS VAF did not significantly differ from VAFs of genes frequently enriched at the time of leukemic transformation, such as IDH (33.7%) (p=0.079) and TP53 (18.2%) (p=0.582) (Fig 1b). The presence of concurrent JAK-STAT activating mutations with RAS mutations was associated with distinct clinical features. RAS mutations occurred more frequently in patients with fibrotic MPN and post MPN AML and were less frequent in patients with ET and PV (table 1). RAS mutant patients had clinical features of more proliferative disease with higher total WBC, absolute monocytes, percentage circulating blasts, higher frequency of splenomegaly, and were associated with higher DIPSS score in pts with myelofibrosis (Table 1).
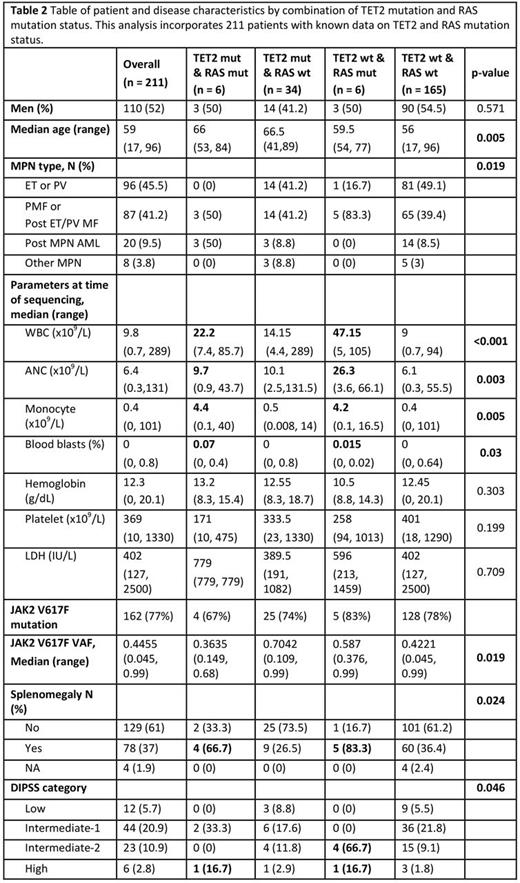
RAS mutations were associated with mutations in TET2 with 4/32 (13%) with mut-TET2 vs 6/152 (4%) wild type TET2 having RAS mutations, though this result did not reach statistical significance (p=0.074) (Fig 1d). No other gene mutation was positively associated with mutations in RAS. 3/6 pts with TET2/RAS co-mutation had post MPN AML, while the remaining 3 had PMF or post ET MF (Fig 1c). The VAF of mut-RAS was always lower than mut-TET2 in patients where these were co-mutated, a pattern not seen for RAS VAF relative to JAK2 V617F VAF. This suggests that RAS mutations were acquired after TET2 in all cases assessed. Patients with RAS/TET2 co-mutation had the highest incidence of post-MPN AML. In those with wild type TET2 and mutant RAS proliferative disease features were noted including high WBC, monocyte count and splenomegaly. Finally, patients with myelofibrosis and RAS mutations were significantly more likely to have high DIPSS scores.
Conclusions:RAS mutations occur in 6% of pts with Philadelphia negative MPN. Patients with co-occurring JAK-STAT pathway activating mutations and RAS mutations had more proliferative disease and a higher incidence of post MPN AML. RAS mutations were more frequent in patients with mutant TET2 and in this context they were associated with post MPN AML. The RAS VAF was always lower relative to TET2 and other MPN driver mutations suggesting they are acquired later in disease evolution. These data suggest the presence of RAS mutations may alter the disease biology of MPNs, and raise the question of the possible clinical efficacy of utilizing therapeutic agents, such as MEK inhibitors, in MPN patients with RAS mutations. Further preclinical and clinical evaluation of the role of this pathway in MPN pathobiology is warranted.
Levine:Qiagen: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal