Abstract
ABSTRACT
Introduction: Systemic mastocytosis (SM) is a complex and rare disease of clonal mast cells. Mast cell leukemia (MCL) is a very rare form of SM seen in <1% patients. We describe here our experience with MCL patients treated at our institution.
Methods: We reviewed the medical records of 218 patients with mastocytosis who presented to our institution between 1994 and 2016. The survival of patients was calculated from the date of initial presentation to the date of last follow up. Kaplan-Meier product limit method was used to estimate the median survival.
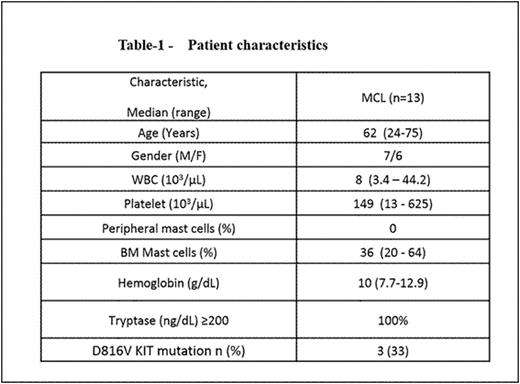
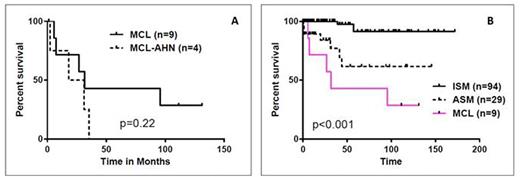
Results: From the 218 patients with mastocytosis, we identified 13 patients with MCL (6.5%). All 13 had aleukemic variant of MCL (aMCL; no presence of mast cells in blood measured by standard CBC technique but with ≥ 20% atypical mast cells in the marrow aspirate). In addition, in 4 of 13 patients, associated hematologic neoplasm (AHN) was present: 2 with myelodysplastic syndrome, 1 with chronic myelomonocytic leukemia and 1 with multiple myeloma. Median age of 13 patients was 62 years (range 24-75 years). Baseline features are summarized in Table-1. During their initial clinical presentation, 85% had constitutional symptoms, 54% had skin rash and other cutaneous symptoms, 31% gastrointestinal symptoms and 23% had joint pains. Imaging studies were performed in 6 patients: 5/6 had enlarged liver and/or spleen, 3 patients had nodal involvement, 2 had sclerotic bony lesions and 1 pt had adrenal involvement. Serum tryptase was >200 ng/ml in all the patients. Testing for c-KITD816V mutation was performed in 9 patients using mutation-specific quantitative (real-time) PCR, of which mutation was undetectable in 6 and detected in 3 patients. The sensitivity of detection was approximately 1 in 1000 mutation-bearing cells. None had FIP1L1-PDGFRA mutations. Treatments given were heterogeneous. Two patients each received imatinib with no response, 2 received dasatinib (one [positive for KITD816V mutation] had significant improvement in hepatosplenomegaly and pruritus but progressed after 1 year of dasatinib therapy), 2 cladribine based therapy with no response, and 2 with stem cell transplant and progression after it. Overall, median follow up was 111 months (1.4-131); 3 patients were alive and 9 died at the time of last follow up. Interestingly, among our 13 aMCL patients, those 4 with AHN appear to have had worse outcome: Figure-1A, shows the median overall survival (OS) of aMCL vs aMCL-AHN (32 vs 25 months; p=0.22). Overall, the median overall survival (OS) of aMCL without AHN was significantly inferior compared to aggressive systemic mastocytosis (ASM) and indolent systemic mastocytosis (ISM) without AHN: 31 months and not reached respectively (Figure-1B; p<0.001).
Conclusions: In this analysis, we have shown that aMCL is very rare and these patients have very poor outcome. Based on the recent data from Gotlib et al NEJM 2016, role of midostaurin in the treatment of MCL should be explored. Further studies to characterize the genomic profile of patients with MCL are needed to identify potential therapeutic targets and disease resistance pathways.
Survival of patients with aleukemic mast cell leukemia (aMCL) with/without AHN (A) and survival comparison of aMCL to other types of systemic mastocytosis, all without AHN (B)
Survival of patients with aleukemic mast cell leukemia (aMCL) with/without AHN (A) and survival comparison of aMCL to other types of systemic mastocytosis, all without AHN (B)
Daver:Sunesis: Consultancy, Research Funding; Kiromic: Research Funding; Ariad: Research Funding; Pfizer: Consultancy, Research Funding; Otsuka: Consultancy, Honoraria; Karyopharm: Honoraria, Research Funding; BMS: Research Funding. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. Verstovsek:Celgene: Research Funding; Bristol-Myers Squibb: Research Funding; AstraZeneca: Research Funding; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Galena BioPharma: Research Funding; Gilead: Research Funding; CTI BioPharma Corp: Research Funding; Lilly Oncology: Research Funding; NS Pharma: Research Funding; Geron: Research Funding; Promedior: Research Funding; Seattle Genetics: Research Funding; Roche: Research Funding; Pfizer: Research Funding; Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal