Abstract
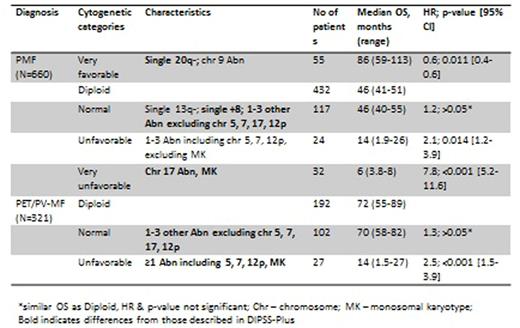
Introduction: The Dynamic International Prognostic Scoring System-Plus (DIPPS-Plus) for primary myelofibrosis (PMF) categorizes cytogenetic abnormalities as "favorable" and "unfavorable." Abnormalities (Abn) -7/7q-; -5/5q-; i(17q), +8; inv(3); 12p-; 11q23; and complex karyotype (CK; >3 Abn) are considered unfavorable. More recently monosomal karyotype (MK), defined as 2 autosomal monosomies or a single monosomy with at least one additional structural Abn, was also recognized as unfavorable (Vaidya, Blood 2011). While the prognostic impact of cytogenetic Abn has been studied in patients with PMF, almost no data exist in patients with post-essential thrombocythemia/polycythemia vera myelofibrosis (PET/PV-MF).
Objective: To identify the impact of cytogenetic Abn on prognosis in patients with PMF and PET/PPV-MF referred to our center between 1984 and 2013.
Methods: We retrospectively reviewed the charts of 1100 patients with MF. Cytogenetic analysis performed at the time of referral to our institution was reported according to the International System for Human Cytogenetic Nomenclature. Overall survival (OS) was calculated and compared using the Kaplan-Meier method with the log rank test. The impact of each cytogenetic Abn on OS was measured by stepwise Cox regression model by comparing them against patients with diploid karyotype. Analyses were conducted separately for patients with PMF and PET/PPV-MF.
Results: Cytogenetic data (≥ 10 metaphases) were available in 981 patients (660 with PMF and 321 with PET/PPV-MF). Median age was similar in both groups (66 years; range, 27-90), and 61% of patients were male. The distribution of DIPSS scores were similar in both groups (overall 7% low, 37% int-1, 41% int-2 and 15% high). OS in each DIPSS category were 134, 65, 33, 19 months in patients with PMF; and 160, 73, 48, 36 in patients with PET/PPV-MF (in both groups P<0.001). The JAK2 mutation was present in 65% of patients in both groups.
Overall, 621 (63%) patients had diploid karyotype (DK), 17% had CK (n=62), and 7% had MK (n=26). Abnormal karyotypes present in >10% of patients were single 20q- (n=75, 21%), single 13q- (n=38, 11%), and CK (n=62, 17%). Others Abn (single +8, +9, single -7/7q-, -5/5q-, or various combinations of the two Abn) occurred less frequently. Importantly, Abn of chromosome (chr) 17 occurred only in patients with PMF, while all other Abn were similarly distributed among PMF and PET/PPV-MF. Ninety nine patients (10%) developed AML, 44% of whom had cytogenetic Abn, similar in PMF and PET/PPV-MF. After a median follow-up of 31 months (range, 0.5-251), 548 (56%) of patients have died, with similar rates in both groups.
Impact of different cytogenetic Abn on OS is presented in Table 1 and Graph 1 for PMF, and Graph 2 for PET/PPV-MF. We have identified 4 different risk categories in PMF patients with respective median OS of 86, 46, 14 and 6 months (P<0.001, Graph 1A). Only 2 different categories were identified in patients with PET/PPV-MF, with the corresponding OS of 70 and 14 months (P<0.001, Graph 1B); further separation of these patients was not possible due to smaller number of patients with specific Abn.
Conclusions: Results from our cohort of 691 PMF and 321 PET/PPV-MF patients differ from those described in DIPSS-Plus. First, we showed that OS in patients with PMF and single +8 Abn, and 1-3 abnormalities (excluding chromosomes 5, 7, 12p and MK) is no different than in those with normal karyotype. Second, OS was the best in patients with single 20q- Abn, which was significantly better than in patients with normal karyotype. Third, we report cytogenetic stratification on the largest cohort of PET/PPV-MF patients, with findings different than in PMF (Table 1). Further validation in larger multicenter studies is warranted.
Overall survival in PMF [1A] and PET/PPV-MF [1B] patients stratified by cytogenetic groups.
Overall survival in PMF [1A] and PET/PPV-MF [1B] patients stratified by cytogenetic groups.
Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

![Figure. Overall survival in PMF [1A] and PET/PPV-MF [1B] patients stratified by cytogenetic groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/22/10.1182_blood.v128.22.4250.4250/3/m_4250fig02.jpeg?Expires=1767737968&Signature=1aXt9uA0w9gqvFcU8D~h0V7yjAbXwQi-7EglA5tO4bhIWhzxPHfjvK-GzTuBqo2oygM~eNc05qdfo1esRy9SYCxBN7cNXeZPA6Jt4dQ4rqwdC3soZcVtbPhg6LTNqgeZyjHkgupGTK~~pVfrpcmX~~QCmB1tkrBNm~qjwl8w9QLu-kBpX0zJFtTG0N6tB7YvlFyySZtAgjoxlXQifw-KAEGwHaga9ceAt62P5cu2Jp21LdbaBAkzharLFNQXk6~ckZRMaYFwRYqyG0~Ad~WAj7X~UQ3yy25uBITl3FeWukgpoj7wqopcHssQw7HMbOKUGMcO0ct5bYIv~vqNzSksTw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal