Abstract
Introduction: Treatment patterns and clinical outcomes in elderly patients with DLBCL treated outside of clinical trials are poorly characterized. A previous report from Medicare Claims data found that 28% of patients above the age of 66 were treated with rituximab monotherapy or were not treated at all, with corresponding median survival of 18 and 2 months, respectively (Hamlin PA et al., Oncologist 2014; 19:1249-57). We conducted a retrospective chart review of diffuse large B cell lymphoma (DLBCL) patients diagnosed and managed within a community oncology practice network to evaluate treatment patterns and corresponding clinical outcomes.
Methods: This was a retrospective observational chart review study of patients aged 60 years and over with newly diagnosed DLBCL. Eligibility criteria required a diagnosis between 1/1/2011 and 12/31/2012, with follow up through 12/31/14, plus no prior therapy for DLBCL. Data were obtained via programmatic query of the US Oncology Network/McKesson Specialty Health electronic health record database. Manual chart review was then performed on a subset of patients (n = 301) to confirm initial findings and gather additional information. Structured data elements were evaluated in univariate and multivariable logistic regression analysis in the subset of patients with stage 2 to 4 disease in order to determine factors associated with treatment with standard R-CHOP. Overall survival (OS) and progression free survival (PFS) were estimated from treatment initiation using the Kaplan Meier (KM) method.
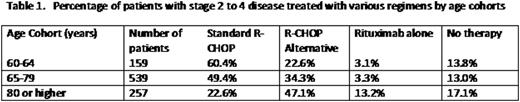
Results: 1151 patients who fit the eligibility criteria were identified. Age significantly influenced frontline treatment selection. Table 1 lists the percentage of patients with stage 2 to 4 disease treated with various regimens by age cohorts. "R-CHOP Alternative" was defined as one of the following: less than 6 cycles of R-CHOP, < 80% of full doses of agents in R-CHOP, or use of an alternative regimen like R-CVP, R-CEOP, or bendamustine-R. In multivariable analysis, age (OR=4.07 for age 65-79 and OR=7.98 for age 60-64; p<0.0001), ECOG 0-1 (OR=2.44, p=0.1380), geographic practice region other than south (OR=1.96, p=0.0002), body mass index (OR=2.52 for underweight/normal and OR=1.93 for overweight, p<0.0001 compared to obese), increased albumin (OR=1.49, p=0.0046), and increased bilirubin (OR=0.56, p=0.0081) were identified as clinically relevant predictors of the likelihood of receiving standard R-CHOP chemotherapy.
Patients with a documented history of cardiomyopathy, congestive heart failure, EF < 50%, chronic renal insufficiency, or diabetes had a reduced prevalence of receiving standard R-CHOP chemotherapy compared with patients with none of those risk factors (34.3% versus 51.2%, p = 0.0640).
Median PFS in the R-CHOP, attenuated R-CHOP, and rituximab monotherapy groups was 51.5 months, 11.0 months, and 8.6 months, respectively. 12- and 24-month OS with standard R-CHOP, R-CHOP alternative, and rituximab monotherapy were 90% and 80%, 78% and 64%, and 74% and 47%, respectively. 2-year OS for patients with IPI 0-2 and IPI ≥3 was 80% and 58%, respectively.
Conclusions: As patients with newly diagnosed DLBCL get older, fewer receive standard R-CHOP chemotherapy. Age, performance status, albumin, bilirubin, cardiac and renal function, and the presence of diabetes mellitus affect ability to receive standard R-CHOP. Aggressiveness of treatment correlates with clinical outcomes.
Burke:Incyte: Consultancy; Janssen: Consultancy; Pfizer: Consultancy; TG Therapeutics: Other: Travel Expenses; Millenium: Consultancy. Black-Shinn:McKesson Specialty Health: Employment. Clark:McKesson Specialty Health: Employment. Frytak:McKesson Specialty Health: Employment, Equity Ownership. Espirito:McKesson Specialty Health: Employment. Sharman:Acerta: Research Funding; Gilead: Research Funding; Celgene: Research Funding; Pharmacyclics: Research Funding; TG Therapeutics: Research Funding; Seattle Genetics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal