Abstract
Background
Novel mechanisms of action (MOA) are needed for the treatment of NHL. Because of the ubiquity and persistence of CD20 expression in B-cell malignancies, there is strong rationale to develop novel MOAs targeting CD20. However, CD20's non-internalizing nature has impeded the development of novel MOAs against this target.. MT-3724 is a recombinant fusion protein consisting of a CD20 binding variable fragment (scFv) fused to the ribosomal inhibitory protein Shiga-like toxin-I A1 subunit (SLT-I A1). Upon scFv binding to surface CD20, SLT-I A1 forces MT-3724 internalization and irreversibly inactivates cell ribosomes triggering cell death. MT-3724 has been shown to specifically bind and kill CD20+ malignant human B-cells in vitro and in in vivo animal models. Data from the first eighteen subjects evaluable for efficacy in the on-going Phase I/Ib monotherapy dose-escalation study of MT-3724 are presented.
Methods
MT-3724 is being tested in a first-in-human, open label, ascending dose study (3 + 3 design) in cohorts of 5, 10, 20, 50, 100, and 75 mcg/kg/dose. Eligible subjects who previously responded to a CD20 MAb containing therapy followed by relapse/recurrence of NHL receive 6 infusions over 2 hours in the first 12 days of a 28 day cycle (first cycle). With continued safety, tolerability and lack of tumor progression, subjects may receive 4 additional 6-dose cycles (21 days) with tumor assessments after cycles 2, 4 and 5. Dose escalation is based on < 33% dose limiting toxicities (DLTs) observed during the first 28 day cycle.
Results
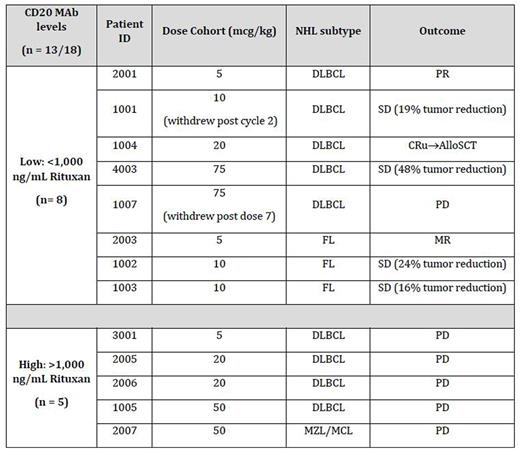
To date, 18 R/R NHL subjects (mean number of prior therapies >4) have enrolled and completed at least one cycle in either the 5, 10, 20, 50, 100, or 75 mcg/kg/dose cohort. Two DLTs were identified in the 100 mcg/kg cohort considered possibly consistent with early signs/symptoms of capillary leak syndrome, a known side effect of immunotoxins. These adverse events (AEs) were non-life threatening and reversible upon drug withdrawal. The most common non-DLT AEs have been reversible hypoproteinemia (≤ Grade 2) with or without transient peripheral edema (≤ Grade 2). A summary of AEs and pharmacodynamic results will be presented. Anti-drug antibodies (ADA) have been observed with MT-3724 but the advent of ADA in subjects has not precluded deepening tumor responses. These data are consistent with the clinical experience of denileukin diftitox, the only approved toxin-based oncology therapeutic. Consistent signs of efficacy including responses were seen in subjects without recent exposure to CD20 antibodies (see table). Conversely, progression by cycle 2 was seen in all subjects who had recent CD20 antibody exposure. CD20 antibodies compete with MT-3724 for target binding and high tissue levels of CD20 antibodies likely inhibit MT-3724 activity.
Conclusions
Targeting CD20 with antibodies has substantially improved survival in NHL, but unmet need remains and there is strong rationale for agents with new MOAs. MT-3724 is the first CD20 targeted immunotoxin to enter clinic trials. Encouraging clinical activity has been seen; safety, efficacy, PK, and ADA data will be presented. Ribosome inhibition represents a novel mechanism of action for the treatment of R/R NHL and continued development of MT-3724 is warranted.
*both Drs. Hamlin and Fanale contributed equally to this work
Hamlin:Molecular Templates: Research Funding; Novartis: Research Funding; Xencor: Membership on an entity's Board of Directors or advisory committees; Portola: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding. Fanale:molecular templates: Research Funding. Valacer:Molecular Templates: Employment, Equity Ownership. Higgins:Molecular Templates: Employment, Equity Ownership. Younes:Molecular Templates: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal