Abstract
Background: Signaling of PI3Kd has been implicated in proliferation, migration, and function of B cells. PI3Kd inhibitors have demonstrated clinical activity in a number of lymphoid tumor types. INCB050465 is a novel, potent, and highly specific inhibitor of PI3Kd (≥19,000-fold more selective for the d vs other isoforms) with no hepatotoxicity in preclinical evaluation at clinically relevant exposures. Here we report the emerging safety, pharmacokinetics, and efficacy results of INCB050465 monotherapy in B-cell malignancies.
Methods: This phase 1/2 study (NCT02018861) enrolled patients aged ≥18 years with relapsed/refractory B-cell malignancies. After an initial single-patient cohort of oral INCB050465 5 mg once daily, a 3+3 dose escalation was conducted with doses ranging from 10 to 45 mg once daily; the dose-limiting toxicity observation period was 21 days. The 20 mg and 30 mg once daily doses were selected for monotherapy expansion. Efficacy was assessed every 9 weeks by Lugano Classification or International Working Group on Chronic Lymphocytic Leukemia (CLL) criteria.
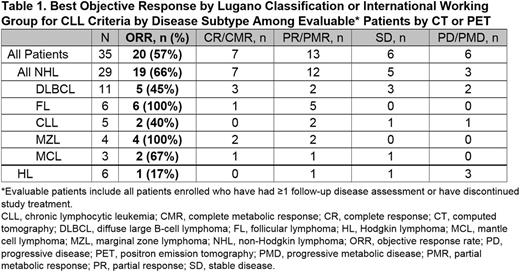
Results: As of the data cutoff (May 20, 2016), 39 patients had been enrolled and treated with INCB050465 monotherapy. The median age was 63 and 62% were men. Subtypes included diffuse large B-cell lymphoma (n=13); Hodgkin lymphoma (HL; n=8); follicular lymphoma (FL; n=6); CLL (n=5); marginal zone lymphoma (MZL; n=4); and mantle cell lymphoma (n=3). At baseline, the median number of prior systemic regimens was 3, and the median time since diagnosis was 4.9 years. INCB050465 demonstrated low oral clearance and linear pharmacokinetics, with a terminal half-life that supports once-daily dosing. The steady-state Cavg was 15-fold greater than the whole blood IC90 at the 30-mg dose. Pharmacodynamic analysis indicated maximal inhibition of the target throughout the dosing interval at all doses tested. Patients received INCB050465 for a median duration of 82 days (range: 4+ to 382+ days); no DLTs were observed. Treatment was discontinued in 16 patients due to disease progression (n=12), adverse events (AEs; n=3), or loss to follow-up (n=1). Dose interruption occurred in 6 patients (15%) and dose reduction in 1 (3%). The most common nonhematologic AEs were nausea (33%), pyrexia (21%), and cough (18%), all of which were grade 1 or 2. All observed alanine aminotransferase (15%) and aspartate aminotransferase elevations (15%) were grade 1. Nine patients (23%) experienced 16 grade ≥3 nonhematologic AEs, 3 of which were considered treatment-related by the investigator. New or worsening grade ≥3 anemia, thrombocytopenia, and neutropenia occurred in 8%, 10%, and 15% of patients, respectively. There was 1 instance (3%) each of colitis (grade 3) and pneumonitis (grade 2). Thirteen patients (33%) experienced a serious AE (SAE); SAEs occurring in more than 1 patient included diarrhea, exfoliative dermatitis, and hypotension (n=2 each). Among efficacy-evaluable patients (n=35), the objective response rate (ORR) was 57% and varied by disease subtype (Table 1). The ORRs for non-Hodgkin lymphoma (NHL) and HL were 66% (19/29) and 17% (1/6), respectively. Objective responses were observed in both transformed and non-transformed DLBCL, and both germinal center B-cell (GCB) and non-GCB subtypes. Objective responses were observed for all 10 patients with FL or MZL. Ninety percent of objective responses were observed at the first response assessment, and responses occurred at all but the 5-mg dose.
Conclusion: The linear pharmacokinetics and absence of DLTs allow INCB050465 to achieve higher levels of target inhibition at the recommended phase 2 dose (30 mg once daily) than have been reported for other PI3Kd inhibitors. INCB050465 monotherapy was well tolerated at all doses tested with no significant transaminase elevations or early-onset diarrhea, and no cases of Pneumocystis jirovecii pneumonia. Objective responses, including complete responses, were observed in both aggressive and indolent NHL. Enrollment is ongoing at the 30 mg once daily dose level.
Gutierrez:Bayer Health Care Pharmaceuticals, Inc.: Other: Traveling and Lodging- Food and Beverage; Pfizer Inc: Consultancy; Merck Sharp & Dohme Corporation: Consultancy, Other: Travel and Lodging; Pharmacyclics LLC, An AbbVie Company: Other: Food and Beverage; Incyte Corporation: Consultancy; E.R. Squibb & Sons, LLC (Bristol Myers Squibb): Consultancy, Other: Travel and Lodging. Edenfield:Astellas/Medivation: Speakers Bureau; Novartis: Speakers Bureau; Greenville Health System Cancer Institute: Employment. Dawkins:Incyte Corporation: Employment, Other: Stocks. DeMarini:Incyte Corporation: Employment, Other: Stocks. Zhou:Incyte Corporation: Employment, Other: Stocks. Yeleswaram:Incyte Corporation: Employment, Other: Stocks. Newton:Incyte Corporation: Employment, Other: Stocks. Chen:Incyte Corporation: Employment, Other: Stocks. Forero-Torres:Seattle Genetics: Research Funding; Genentech/Roche: Research Funding; Juno: Research Funding; Incyte: Research Funding; Abbvie: Research Funding; Novartis: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal