Abstract
Background
OSis the gold standard primary efficacy endpoint for evaluating treatment strategies for DLBCL. Because current standard of care first-line regimens with rituximab plus chemotherapy produce median OS > 8 years, an endpoint assessed earlier would accelerate the evaluation of new therapies and potentially allow patients (pts) to more rapidly benefit from study results. The SEAL group formally evaluated PFS as a surrogate endpoint for OS in first-line therapy for DLBCL.
Methods
Through a systematic literature search and input from lymphoma experts on the SEAL committee, we identified multicenter, randomized controlled clinical trials that evaluated active treatment and enrolled at least 100 pts with previously untreated DLBCL or aggressive non Hodgkin lymphoma by WHO/REAL classification, published after January 1, 1995. Studies which enrolled only early stage (I or II) pts and studies that could not provide individual pt data (IPD) were excluded from surrogacy analysis. Potential surrogate candidates for OS included: 1) PFS, defined as the time from initiation of induction treatment to progressive disease, relapse or death due to any cause, and 2) PFS rate at 24 months after initiation of induction treatment (PFS24). These endpoints were derived consistently for all studies based on IPD. Surrogacy was evaluated by the correlation between surrogate candidates and OS. Pt-level correlation was measured by the rank correlation coefficient (ρ) and global odds ratio for PFS and PFS24, respectively, estimated by the Copula model. Trial-level correlation was evaluated by the correlation between trial-specific treatment effects on surrogate candidates and OS with two commonly used trial-level R2s: R2WLS (Sargent et al JCO 2005) and R2Copula (Burzykowski et al J R Stat Soc C 2001 and Burzykowski et al J R Stat Soc A 2004). For the R2 measures, a value closer to 1 indicates a stronger correlation. We pre-specified that if either trial-level R2 was >=0.80 and its 95% confidence interval (CI) excluded 0.60, the candidate would meet criteria for surrogacy.
Results
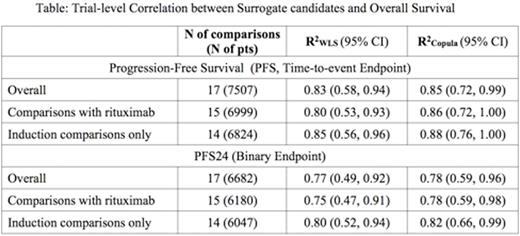
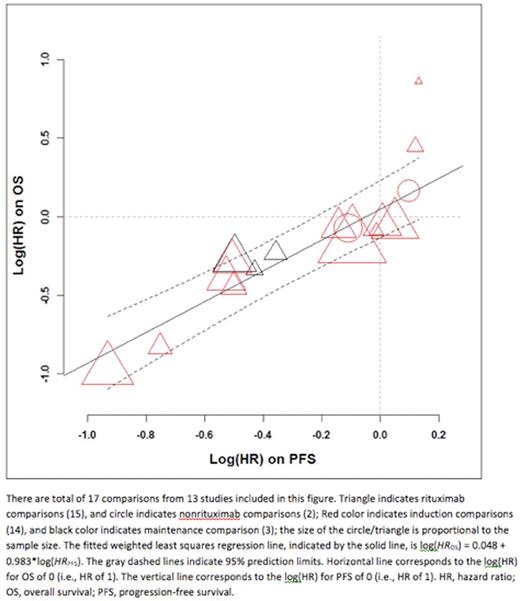
Thirteen of 14 studies collected to date in the SEAL database met all inclusion criteria. IPD for 7507 pts (median age 63 years, 54% male, 46% ECOG performance status 0, 8% IPI 0, 50% IPI 1-2, 64% stage III/IV, median follow-up time of 52 months) were analyzed. Among 13 studies, one tested maintenance treatment only and two tested both induction and maintenance regimens. One study included four treatment arms. Thus at the trial level, 17 two-arm comparisons were formed: 14 comparisons comparing induction regimens and 3 comparing maintenance; 15 comparisons involved rituximab. Longer PFS was highly correlated with longer OS at the pt level (ρ= 0.85, 95% CI 0.84-0.86), as was PFS24 (Global odds ratio = 61.1, 95% CI 52.6-69.6). The Table shows trial-level correlations, and the Figure shows trial-level association between estimated treatment effects (log(HR) [hazard ratio]) on PFS and OS. The trial-level correlation between PFS and OS was strong and met the pre-specified surrogacy criteria, with R2WLS of 0.83 (95% CI 0.58-0.94) and R2Copula of 0.85 (95% CI 0.72-0.99). Sensitivity analyses including only comparisons including rituximab or only induction regimens showed consistent results. The overall trial-level correlation between PFS24 and OS was reduced slightly, with R2WLS of 0.77 (95% CI 0.49-0.92) and R2Copula of 0.78 (95% CI 0.59-0.96). Performance of PFS24 was improved when restricting analysis to only including induction studies (see table).
Conclusion
This large IPD pooled-analysis demonstrates that PFS duration met the pre-specified criteria as a robust surrogate endpoint for OS, and can be considered as a surrogate endpoint for OS for frontline DLBCL trials evaluating immunochemotherapy. The earlier endpoint of PFS24 showed promise as a potential surrogate and further validation is warranted.
Shi:Mayo Clinic: Employment. Flowers:Millenium/Takeda: Research Funding; TG Therapeutics: Research Funding; Pharmacyclics, LLC, an AbbVie Company: Research Funding; Gilead: Consultancy, Research Funding; NIH: Research Funding; Mayo Clinic: Research Funding; ECOG: Research Funding; Acerta: Research Funding; Infinity: Research Funding; AbbVie: Research Funding; Roche: Consultancy, Research Funding; Genentech: Consultancy, Research Funding. Ou:Mayo Clinic: Employment. Cunningham:Merrimack: Research Funding; Celgene: Research Funding; Bayer: Research Funding; Astra-Zeneca: Research Funding; Amgen: Research Funding; Medimmune: Research Funding. Pfreundschuh:Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Spectrum: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Seymour:AbbVie Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Jaeger:Roche: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Haioun:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sandoz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees. Ghesquieres:Mundipharma: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche France: Research Funding. Merli:Teva Pharmaceuticals Industries: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees. Herbrecht:Cell Therapeutics, Inc: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees. Flament:Celgene: Employment, Equity Ownership. Fu:Celgene: Employment, Equity Ownership. Coiffier:MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Sargent:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal