Abstract
Introduction
Primary CNS lymphoma (PCNSL) is a diffuse large B-cell lymphoma (DLBCL), predominantly of non-germinal center (non-GC) subtype. Currently available treatments remain disappointed with a non-optimal complete remission rate and a high relapse rate. As such, novel therapeutic agents are urgently needed. Constitutive activation of the NF-kB pathway via mutations in B cell receptor (BCR) (CD79B) and mutation of MYD 88 and TBL1XR1 pathways plays an important role in PCNSL. Ibrutinib, an inhibitor of BCR signaling, has been found to have significant therapeutic activity in relapsed or refractory non-CNS non-GC DLBCL that display similar NFKB mutational patterns. We have performed a CNS pharmacokinetic (PK) study of Ibrutinib and evaluated its therapeutic activity against CNS lymphoma (CNSL) in murine models.
Methods
CNS PK study in mice - The first study was done in a CNSL model created by intracerebral implantation of luciferase-transfected lymphoma cells (MC116) in nude mice in comparison to healthy nude mice without lymphoma cell implantation (N=5 per group). Once the tumor activity was confirmed by bioluminescence imaging in CNSL group, all the mice were treated with ibrutinib 12 or 30 mg/kg/day by oral gavage (OG) for 5 days. Blood and brain were collected 2 hours after the last dose. Ibrutinib was measured by ultra performance liquid chromatography with tandem mass spectrometry.
Cerebro-spinal fluid (CSF) PK study in Sprague-Dawley (SD) rats - Ibrutinib 50 mg/kg single dose was given by OG (N=6). Microdialysates of CSF were collected from a ventricle of the brain and a carotid artery via microdialysis catheters every hour for 13 hours post-treatment. Drug concentrations were determined by capillary electrophoresis with laser detection.
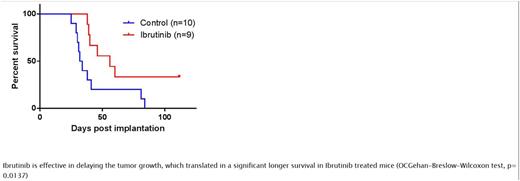
Preclinical therapeutic evaluation - A murine CNS lymphoma model was created by intracerebral injection of the OCI-Ly10 non-GC DLBCL cells, which were confirmed to have CD79 A and MYD 88 mutations and show high sensitivity to Ibrutinib in vitro. Animals were sacrificed if they showed severe clinical signs or symptoms or >20% weight loss. Ibrutinib 30 mg/kg was administered by OG daily starting at day 7 post-implantation and until the last sacrifice in the control group treated with vehicle. Therapeutic efficacy was determined by Kaplan-Meier survival analysis using date of sacrifice as endpoint.
Results
CNS PK study in mice - 1) Ibrutinib levels in plasma, normal brain tissue and brain-tumor were analyzed. Ibrutinib was not detected in healthy control mice treated with vehicle. In healthy mice treated with ibrutinib (12 mg/kg and 30 mg/kg), Ibrutinib mean plasmatic levels were 9.31 (±3.56) ng/mL and 42.2 (±20.9) ng/mL respectively. At 12 mg/kg dose level, ibrutinib was detectable but not quantifiable in brain tissue. After treatment with 30 mg/kg,iIbrutinib mean levels were 0.0077 (±0.0053) and 0.0071 (±0.00483) ng/g tissue in right and left cerebral hemispheres respectively. In CNSL mice treated with ibrutinib (12 mg/kg and 30 mg/kg), Ibrutinib mean plasmatic levels were 12.03 (±9.04) ng/mL and 42.64 (±42.04) ng/mL respectively, brain ibrutinib levels were 0.008 (±0.005) and 0.007 (±0.003) ng/g tissue for tumor-bearing right hemisphere and non-tumor bearing left hemisphere respectively for 30 mg/kg, while brain ibrutinib level was detectable but not quantifiable at 12mg/kg.
CSF PK study in SD rats - CSF penetration of ibrutinib was determined to be ~5.2 % as calculated by area under the curve (AUC) ratio of CSF and blood samples. Cmax in CSF is ~0.35 uM, which is significantly higher than published EC50 concentrations for DLBCL cell lines.
Preclinical therapeutic evaluation -Ibrutinib showed an excellent therapeutic activity with significant prolongation of survival (p=0.0137) (fig 1). At a median follow-up of 112 days, the median survival in treated CNSL group is 56 days compared to 33 days in the control group. At the end of the follow-up 33% of animals in treated CNSL group are still alive without treatment.
Conclusion
Ibrutinib achieves high concentrations in brain and CSF in murine CNS PK models with promising single-agent preclinical therapeutic activity against CNS lymphoma in a murine model. Further studies to develop combination regimen are ongoing.
Soussain:Roche: Research Funding; Celgene: Research Funding; Pharmacyclics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal