Abstract
Treatment of relapsed/refractory peripheral T-cell lymphoma (R/R PTCL) remains an unmet medical need, as treatment with polychemotherapy is associated with poor outcome. With the exception of Brentuximab vedotin in systemic anaplastic large cell lymphomas, recent FDA approved drugs to treat R/R PTCL showed limited activity, with a 30% overall response rate (ORR), a complete response (CR) rate of 10-15% and short remission duration.
Recurrent mutations of TET2 or other genes involved in DNA methylation regulation have recently been described in PTCL, especially in angioimmunoblastic T cell lymphomas (AITL) where TET2 mutations are present in 50-70% of patients. These molecular anomalies are also present in myeloid malignancies, which can be sensitive to treatment with hypomethylating agent. However, efficacy of hypomethylating agent in PTCL is unknown.
We report here the results of 5-Azacytidine (5-AZA) treatment in 19 R/R PTCL patients. Two of them have been previously published (Cheminant et al. BJH 2015; Saillard et al. Hematol Oncol 2016).
Treatment was administered between January 2013 and July 2016 according to physician decision and consisted in subcutaneous injection of 75 mg/m² 5-AZA during 7 consecutive days every 28 days, until progression or unacceptable toxicity. Six patients received rituximab for 4-8 infusions because of EBV-DNA positivity. Evaluation was performed locally according to IWG guidelines.
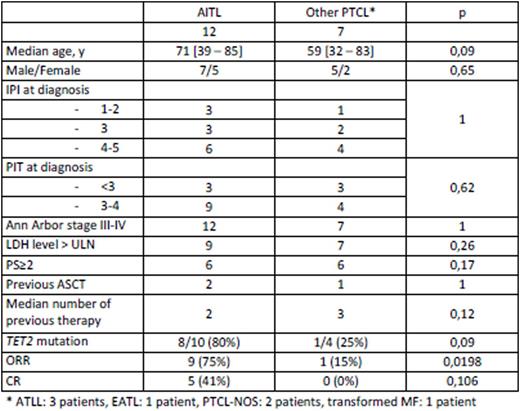
Baseline characteristics of patients are presented in table 1. All patients presented with adverse prognostic factors and 17 patients had R/R PTCLs. Ten patients had previous or concomitant diagnosis of myelodysplastic syndrome, mostly (9/10) chronic myelomonocytic leukemia (CMML). Two patients did not receive chemotherapy before 5-AZA because of the presence of an associated CMML that required treatment first for myeloid malignancy, according to physician decision.
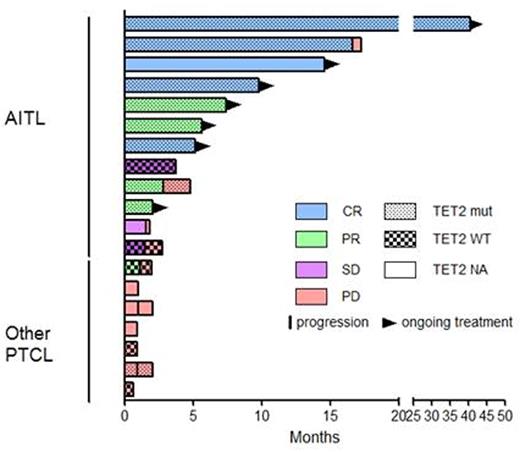
Patients received a median number of 3 cycles of 5-AZA [1 - 43] and, at time of analysis, 7 patients are under therapy (Figure 1). Hematological toxicity was as expected. Two patients developed unusual adverse reaction: grade 2 polyneuropathy (which was deemed to be related to paraneoplastic syndrome) and grade 3 diarrhea of unknown origin, the latest leading to treatment interruption.
The median follow-up was 84 days [19 - 1236]. Overall response rate (ORR) for the entire population was 53% (10/19), but was significantly higher in AITL patients than in patients with other PTCL entities (9/12, 75% vs. 1/7, 15%, p=0,0198). Among these 10 responding patients, 5 patients - all suffering from AITL - reached complete response (CR), leading a CR rate of 42% in this entity. Three additional patients were in stable disease (best response) and 6 progressed rapidly. It is noteworthy that among the 9 AITL patients who responded, only 2 patients experienced progression after 86 and 499 days, respectively, the remaining 7 patients being still responders and under therapy. None of the patients with another PTCL entity experienced response, with the exception of one patient with ATLL for whom 5-AZA induced a rapid and profound decrease in lymphocyte count; however, this patient relapsed after the second cycle.
TET2 was sequenced in 14 patients and was mutated in 8/10 (80%) AITL and 1/4 (25%) other PTCL. Interestingly, 8/8 AITL patients with TET2 mutational status available who experienced a response after 5-AZA treatment were TET2 mutated.
In summary, 5-AZA treatment is associated with significant response rate in R/R AITL, including prolonged CR. Additional genomic analyses are ongoing and will be presented during the meeting, aiming to correlate response rate with mutational status in the TET2, IDH2 and DNMT3A epigenetic modifier genes. Finally these results warrant further prospective studies evaluating 5-AZA treatment in R/R AITL or R/R PTCL bearing mutations in epigenetic modifiers.
Dupuis:janssen: Honoraria; ABBVIE: Membership on an entity's Board of Directors or advisory committees. Sarkozy:Janssen Research & Development: Honoraria; Sandoz: Research Funding; Takeda: Research Funding; Gilead: Honoraria. Morschhauser:Celgene: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Janssen: Honoraria; Servier: Consultancy, Honoraria. Hermine:AB science: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding, Speakers Bureau; Celgene: Research Funding; Alexion: Research Funding; Novartis: Research Funding. Haioun:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sandoz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal