Abstract
Background:T cell lymphomas account for 10-15% of lymphoid malignancies and display significant heterogeneity. T cell lymphomas have a worse prognosis than most B cell lymphomas. For relapsed or refractory disease, there is not a standard treatment and median progression free and overall survival rates have been reported as 3.7 and 6.5 months, respectively. Therefore, optimal treatment for relapsed/refractory T cell Non-Hodgkin Lymphoma (NHL) is an unmet clinical need.
CPI-613 is a lipoate derivative that has shown activity in hematologic malignancies. CPI-613 selectively targets the pyruvate dehydrogenase complex (PDC) and α-ketoglutarate dehydrogenase complex (KGDHC) in tumor cells. CPI-613 leads to the inhibition of the catalytic and regulatory functions of the PDC and the KGDHC causing alterations of mitochondrial enzyme complex activities and altering redox status, leading to apoptosis, necrosis and autophagy of tumor cells. The anti-tumor activity of CPI-613 is evident in various cancer cell lines, xenograft animal tumor models and clinical trials against a diverse group of cancers. Patients tolerate CPI-613 as a single agent at doses up to 3,000 mg/m2, according to Phase 1 trials in patients with solid tumors and hematologic malignancies. Bendamustine has shown single agent activity in the relapsed lymphoma setting with response rates of approximately 50% for T cell NHL. Here, we are conducting a Phase I study in which the primary objective was to evaluate the maximum tolerated dose (MTD) of CPI-613 while administered in combination with Bendamustine. The secondary objective was to determine response rate to treatment.
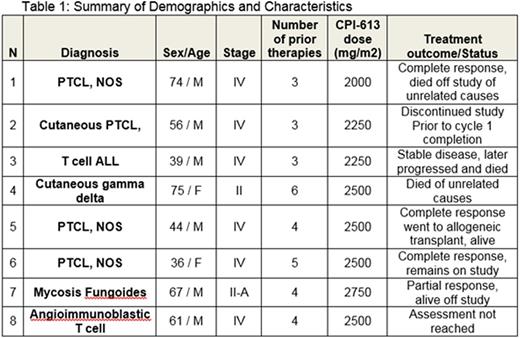
Methods: This study is a phase 1, open label, modified 3+3 dose escalation clinical trial evaluating CPI-613 and Bendamustine combination therapy. CPI-613 was given at escalating doses starting at 2,000mg/m2 over 2 hrs on days 1-4, and on days 8, 11, 15 and 18. Bendamustine was infused at 90 mg/m2 on days 4 and 5 of each treatment cycle. Each treatment cycle was 4 weeks and repeated up to six cycles. Demographics, patient characteristics and dose levels are shown in the table below. There was no intra-patient dose escalation.
Results: As of July 27, 2016, eight subjects have received at least one dose of study drug. Eight patients are evaluable for safety and five patients are evaluable for response. The most common grade 3 or higher toxicities were lymphopenia and neutropenia and occurred in 4 subjects. One patient dosed at 2750 mg/m2 had a dose limiting toxicity of grade 3 acute kidney injury and grade 4 lactic acidosis. The protocol was later amended to discontinue dose escalation at doses of 2750 mg/m2 or higher and to expand the 2500mg/m2 cohort.
The overall response rate was 80%. Three patients with peripheral T cell lymphoma, NOS, obtained a complete response and 1 patient with mycosis fungoides had a partial response. One patient with T cell acute lymphomoblastic lymphoma had progressive disease. The median time to response is 1.8 months. Enrollment is ongoing and updated trial results will be presented.
Conclusions: This first reported study of CPI-613 administration in combination with Bendamustine in subjects with relapsed or refractory T cell lymphoma showed a good safety profile and an excellent overall response rate of 80% with CRs in all three patients with peripheral T cell lymphoma, NOS. Although numbers are small, continued investigation is warranted as these response rates in a poor risk population of patients with relapsed/refractory T cell lymphoma are very exciting.
Clinical trial: NCT02168907
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal