Abstract
Peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS) is the largest subgroup with dismal prognosis in peripheral T-cell lymphomas (PTCL) that do not fit into any of the specifically defined categories in the World Health Organization classification, and it comprises over 25% of PTCL. PTCL-NOS is sometimes referred to as the gwaste-basketh category because of the lack of specific features, and therefore contains heterogeneous disease entities. Its heterogeneity represents a major obstacle to elucidation of pathogenesis, development of novel therapeutic targets and establishment of standardized therapeutic strategies and results in its poor prognosis.
The stratification strategy of PTCL-NOS still remains controversial, although previous studies tried to reclassify PTCL-NOS from the viewpoint of gCell-of-Originh of tumor cells (e.g. T helper 1/2 cells, regulatory T cells) by utilizing histopathologic methods or gene expression profiling. These situations prompted us to investigate microenvironment profile of PTCL-NOS and to develop the novel stratification strategy.
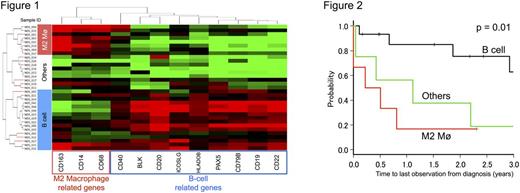
For this purpose, we analyzed the expression levels of 800 of tumor microenvironment-related genes including the phenotypic markers, transcription factors and functional molecules of microenvironment cells in formalin fixed paraffin embedded (FFPE) samples derived from 33 PTCL-NOS patients utilizing nCounter system, which is novel RNA counting system based on a digital molecular barcoding technology and enables accurate quantification of genes with low expression levels. Thirty-three patients who were diagnosed as PTCL-NOS between 1993 and 2011 were included in this study. The median follow-up time was 796 days (range, 15-2973). The overall survival rate at 1 year after diagnosis (1yr OS) was 40%, and it was comparable to previous reports. Unsupervised hierarchical clustering analysis revealed three distinct clusters based on the gene expression pattern of the microenvironment-related genes: B-cell type (42.4 %), M2 macrophage type (24.2 %), and others (33.3 %) (Figure 1). On the other hand, the expression patterns of the major T-cell subset-associated genes were complicated and diverse depending on patients, and failed to form any distinct clusters. B-cell-related genes, such as CD19, CD79B and PAX5 were highly expressed in the B-cell type, and the M2 macrophage-related genes, such as CD68, CD163, CD14 and MARCO were highly expressed in the M2 macrophage type. More importantly, these microenvironment-based subgroups, B-cell type and M2 macrophage type, represent prognostically favorable and unfavorable subgroups, respectively (1yr OS: B-cell type, 84.8%; M2 macrophage type, 16.7%; others, 56.2%; log rank test, p = 0.01) (Figure 2). Interestingly, genes associated with immune checkpoint, such as PD-L1, TIM-3 and IDO-1, were highly expressed in the M2 macrophage type than the others (PD-L1, p = 0.02; TIM-3, p = 0.05; IDO-1, p < 0.01), indicating the existence of specific subgroup of PTCL-NOS patients as anticipated good-responder for immunotherapeutic agents including immune checkpoint inhibitors. Collectively, our findings strongly suggest that microenvironment immune signature, not gCell-of-Originh of tumor cells, could provide novel prognostic stratification method and therapeutic strategy of PTCL-NOS.
Akashi:Asahi Kasei Pharma Corporation: Research Funding; Shionogi & Co., Ltd: Research Funding; Astellas Pharma: Research Funding; Celgene: Research Funding; Kyowa Hakko Kirin: Consultancy, Research Funding; Sunitomo Dainippon Pharma: Consultancy; Bristol Meyers Squibb: Research Funding; Chugai Pharmaceutical Co., Ltd.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal