Abstract
Background. Mantle Cell Lymphoma (MCL)represents an aggressive lymphoma for which an effective treatment has still to be determined. The FIL-MCL0208 phase III trial (EudraCTNumber: 2009-012807-25) is exploring R-CHOP followed byi) high-dose cytarabine, autologous stem cell transplantation, and ii) randomization between lenalidomide maintenance vs observation (Cortelazzo et al, EHA 2015). We performed genome-wide DNA profiling to identify unbalanced copy number variations (CNVs) with a clinical significance in patients enrolled in the FIL-MCL0208 phase III trial.
Patients and Methods. The study included untreated, advanced stage MCL patients (<65 years) enrolled into the MCL0208 trial. Clinical results of the first interim analysis (without random unblinding) have been already reported and are the basis of the present analysis: median follow-up of alive patients was 26 months, and at 2-years, 79% of patients were progression free and 91% alive (Cortelazzo et al EHA 2015). DNA profiling with the Illumina HumanOmni2.5 arrays was performed on 174 DNA samples derived from baseline bone marrow CD19+ purified tumor cells. Genomic profiles were segmented with the Fast First-derivative Segmentation Algorithm (Rinaldi et al, BJH 2013; Kwee et al, bioRxiv 2016). Recurrent CNVs were defined applying both the minimal common regions and the GISTIC algorithms (Lenz et al, PNAS 2008; Mermel et al, Genome Biol 2011). CNVs were integrated with somatic mutational data obtained for 8 genes recurrently affected in MCL (ATM, BIRC3, CCND1, KMT2D, TP53, TRAF2, WHSC1, NOTCH1) (Rossi et al, ASH 2015). Progression free survival (PFS) was the primary endpoint of the analysis.
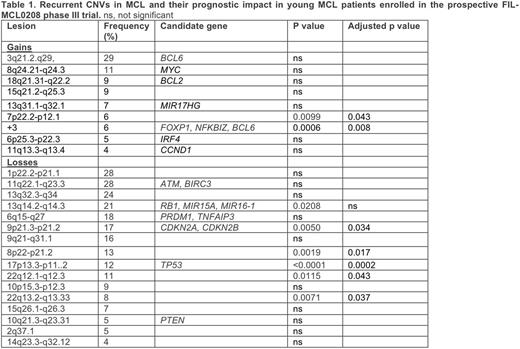
Results. 161 patients were currently evaluable for CNVs and clinical outcome. Patients had an intermediate/high-risk MIPI and a Ki67 ³ 30% in 43% and 42% of the cases, respectively. Twenty-five recurrent unbalanced CNVs were defined (Table 1). By multiple test corrected univariate analysis, seven CNVs had a negative impact on PFS: +3, 7p gain, 9p loss (CDKN2A), 17p loss (TP53), 8p loss, and 2 losses at 22q (Table 1). MIPI (intermediate/high vs low risk), Ki67+ and the TP53/KMT2D model (Rossi et al, ASH 2015) were also statistically significant. Combining CNVs and mutations, TP53 was inactivated in 27/147 cases (18%): mutated/deleted in 7/147 (5%), deleted but not mutated in 14/147 (10%), and mutated but not deleted in 6/147 (4%). The negative prognostic impact was equal for all the 3 inactivation modalities, which were then considered as a single group for further analyses. ATM was inactive in 69/147 (47%): mutated/deleted in 24/147 (16.3%), deleted in 12/147 (8%), and mutated in 33/147 (22.4%). KMT2D (MLL2) was inactive in 18/147 (12%): mutated/deleted in 1/147 (<1%), deleted in 1/147 (<1%), and mutated in 16/147 (11%).
The lesions with a significant impact at univariate were included in a multivariate analysis alongside MIPI, KMT2D inactivation and Ki67+ as continuous variable. Only TP53 inactivation by deletion and/or mutation, 7p22.2-p12.1 gain and KMT2D inactivation maintained their independent prognostic significance.
Conclusions. Genome wide DNA profiling in the FIL-MCL0208 phase III trial identified lesions, namely TP53 and KMT2D inactivation and gains at 7p, which maintain a poor outcome significance in young MCL patients even following high-dose cytarabine and autologous stem cell transplantation.
Rossi:Gilead: Honoraria, Research Funding; Abbvie: Honoraria; Janseen: Honoraria. Stelitano:Azienda Ospedaliera: Employment. Gaidano:Janssen: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; Morphosys: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Speakers Bureau; Karyopharm: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal