Abstract
Background: Pts with AML-Mo, often present with leukocytosis and aggressive clinical features such as extramedullary involvement and coagulation abnormalities. Frequently the clinical presentation resembles a hyperinflammatory phenotype. This often leads to unfavorable outcomes compared to pts with other subtypes of AML regardless of pretreatment cytogenetic/molecular features. No large series have systematically assessed the characteristics of pts with AML-Mo following standard induction therapy. We conducted a retrospective case-control study of pts with AML-Mo to define disease characteristics and associate those with outcome.
Methods: Sixty-one consecutive pts between January 2010 and March of 2016 with AML who met FAB criteria for acute myelomonocytic or acute monocytic leukemia or exhibited greater than 20% monocytic differentiation on flow cytometry were considered as AML-Mo, and were compared to a control cohort of 128 pts with AML without monocytic differentiation. All pts were newly diagnosed and treated on a standard intensive induction protocol. Pts were excluded for incomplete cytogenetic and molecular data.
Results: There were no significant differences between the two groups with regard to age, sex, secondary AML, prior MDS, and ELN risk stratification. Pts with AML-Mo had higher median WBC (43,000/µ vs 7,000/µL, p<0.0001) and higher LDH values (1,661 U/L vs 847 U/L, p<0.0001). The treatment course of pts with AML-Mo was characterized by significantly higher incidence of tumor lysis syndrome (42%vs 6%, p<.0001), diffuse alveolar injury (10% vs 0%, p=.001) , respiratory failure with need for high flow oxygen or mechanical ventilation (31% vs 6%), p=<.0001), symptomatic leukostasis ( 7% vs 1%, p=.014), renal failure (41% vs 10%, p=<.0001), and DIC (19%vs 3%, =.0008). Induction mortality was 18% for AML-Mo and 2% for other AML pts (p=.0003). CR rate was 79% (AML-Mo) and 82% (AML), respectively (p=.65).
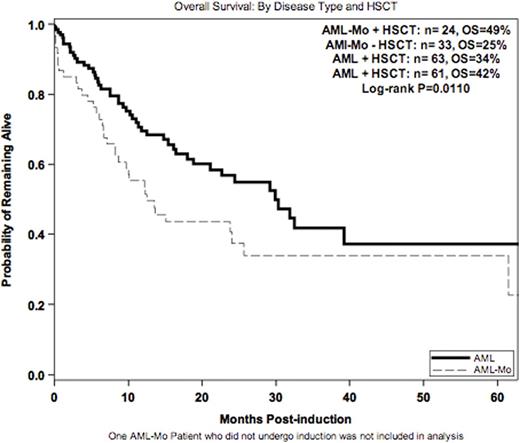
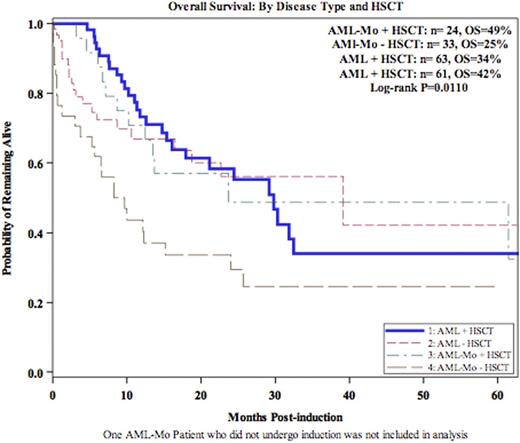
With a median follow up of 10.7 months, 1 and 5-yr OS was 55% and 34% (AML-Mo) compared to 70% and 38% (AML) at 1 and 5 years, respectively (p=.0075, p=.034)(Figure 1). Allo HSCT in CR1 resulted in greater reduction in the risk of mortality for pts with AML-Mo compared to other AML pts (56% vs 11%, p=0.148) (Figure 2). This effect prevailed when considering ELN risk at diagnosis and HSCT into a multivariate model (p=0.032).
Conclusions: Pts with AML-Mo experience higher rates of complications and death within 30 days of induction. Despite similar CR rates, overall survival was inferior. While allo HSCT benefited both pts with AML and AML-Mo, the benefit was greatest for AML-Mo pts. Besides allo HSCT in CR, other measures (eg reduction of inflammatory reactions by suppression of cytokines and other mediators) should be considered to decrease the high induction mortality and complication rate of these pts.
McCloskey:Novartis: Speakers Bureau; Incyte: Consultancy; Ariad: Consultancy, Speakers Bureau; Amgen: Speakers Bureau. Depadova:Amgen: Speakers Bureau. Koprivnikar:Alexion: Consultancy, Speakers Bureau. Stanislaus:Novartis: Consultancy, Speakers Bureau. Goldberg:Neostem: Equity Ownership; Pfizer: Honoraria; Bristol Myers Squibb, Novartis: Speakers Bureau; Novartis: Consultancy; COTA Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal