Abstract
Background: Clinical trial eligibility criteria balance identifying a specific patient population who may benefit from an intervention with restricting allowable comorbidities to maximize safety and avoid off-target drug effects. Patients may also be deemed ineligible for a trial for reasons that do not directly impact efficacy or safety. We identified and categorized reasons for ineligibility and compared outcomes of ineligible patients with eligible patients treated on SWOG Leukemia Committee protocols.
Methods: Patients enrolled in SWOG Leukemia Committee phase II, II/III, or III protocols open since 2005 were analysed for eligibility status, reasons for ineligibility, Eastern Cooperative Oncology Group (ECOG) performance status, serious adverse events (SAEs), and outcomes, including complete remission (CR) status (as defined for each leukemia subgroup by international standards) and overall survival (OS), measured from time of study enrollment. Ineligible patients included in the studies' analyses were analyzed separately from patients excluded from analyses. Reasons for ineligibility are summarized descriptively, and logistic or Cox regression analyses accounting for clustering of outcomes within each study were performed. Multivariable analyses accounted for age, sex, trial design (randomized vs. single-arm), and disease-type (de novo vs. relapsed/refractory).
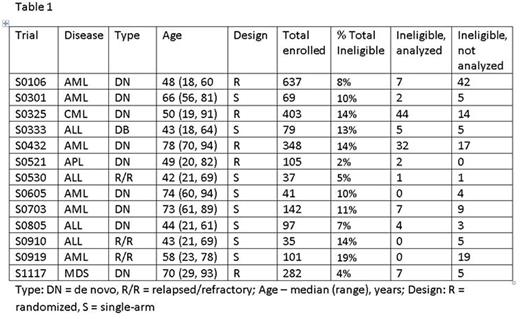
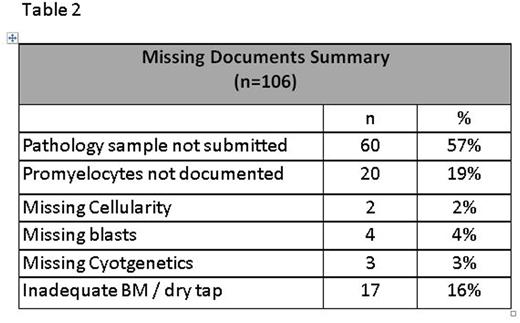
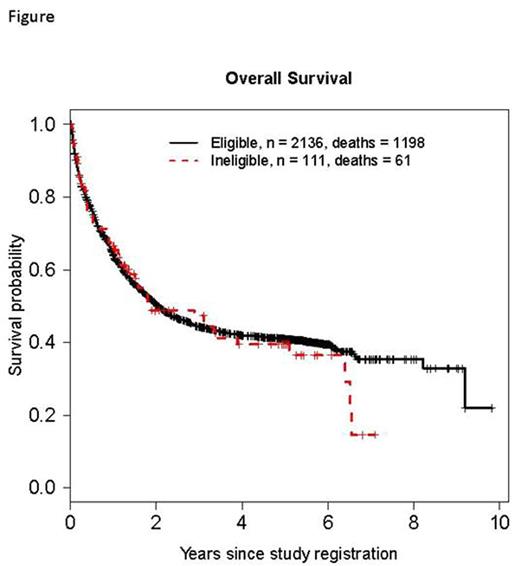
Results: Pooled data (n=2,376) from 13 SWOG trials (S0106, S0301, S0325, S0333, S0432, S0521, S0530, S0605, S0703, S0805, S0910, S0919, S1117) open to accrual since 2005 were included in analyses (Table 1). Of these, 10 were phase II (77%), 2 (15%) were phase III, and 1 (8%) was phase II/III. Most studies (10/13, 77%) included patients with de novo disease: acute myeloid leukemia (AML, n=5), chronic myeloid leukemia (n=1), acute lymphoblastic leukemia (ALL, n=2), acute promyelocytic leukemia (APL, n= 1), and myelodysplastic syndromes (MDS) / chronic myelomonocytic leukemia (n=1). The remainder (4/13, 31%) enrolled previously treated patients: ALL (n=3), and AML (n=1). Across the 13 studies, 257 patients (11%) were deemed ineligible: 81 were excluded from analyses (77% of whom had a different disease than the one being studied or did not meet diagnostic criteria), and 176 were included in all analyses. The most common reasons for ineligibility among these 176 patients were missing documentation (106/176, 60%) and out of window laboratory values (28/176, 16%) or bone marrow biopsy (15/176, 9%). All patients ineligible due to out of window laboratory values did not meet the requirement of having a WBC ≤30,000/cmm within 1 day of registration on S0432, which studied schedules and dosing of tipifarnib in AML patients 70 years or older. The most common missing documentation factors leading to ineligibility were: specimens required for correlative studies (60/106, 57%) and enumeration of bone marrow promyelocytes (20/106, 19%) in non-APL, AML trials (Table 2).Comparing ineligible to eligible patients, ECOG performance status was similar (p=.29) as were the odds ratios for grades 4 and 5 SAEs: 0.74, 95% CI (0.50, 1.11), p=.15 and 0.72, 95% CI (0.17, 3.03), p=.66, respectively. Efficacy outcomes were also similar for ineligible and eligible patients: odds ratio for complete response (CR) = 0.77, 95% CI (0.48, 1.24), p=.28; and the hazard ratio for overall survival (OS) = 0.92, 95% CI (0.74, 1.14), p=.43 (Figure).
Conclusions: The majority of patients ineligible for SWOG Leukemia / MDS studies are either missing documentation or have laboratory results outside of a protocol's timeframe. Despite this, there were no significant differences in ECOG performance status, SAEs (≥ grade 4), CR rates, or survival between ineligible and eligible patients. The findings of this study suggest that eligibility criteria and specific documentation requirements can be less restrictive, thus expanding patient enrollment and avoiding protocol deviations.
Othus:Glycomimetics, Celgene: Consultancy. Erba:Gylcomimetics: Other: DSMB; Millennium Pharmaceuticals, Inc.: Research Funding; Jannsen: Consultancy, Research Funding; Incyte: Consultancy, DSMB, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Amgen: Consultancy, Research Funding; Juno: Research Funding; Celgene: Consultancy, Speakers Bureau; Seattle Genetics: Consultancy, Research Funding; Celator: Research Funding; Pfizer: Consultancy; Astellas: Research Funding; Ariad: Consultancy; Agios: Research Funding; Daiichi Sankyo: Consultancy; Sunesis: Consultancy. Radich:Bristol-MyersSquibb: Consultancy; Pfizer: Consultancy; Novartis: Consultancy, Other: laboratory contract; ARIAD: Consultancy; TwinStrand: Consultancy. Coutre:AbbVie: Research Funding; Janssen: Consultancy, Research Funding; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Research Funding. Advani:Blinatumomab: Research Funding; Pfizer Inc.: Consultancy, Research Funding. Mukherjee:Ariad: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding. Sekeres:Celgene: Membership on an entity's Board of Directors or advisory committees; Millenium/Takeda: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal