Abstract
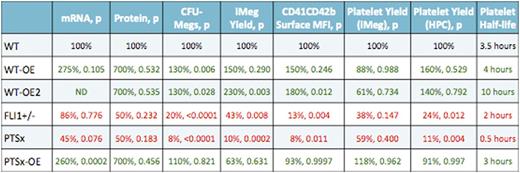
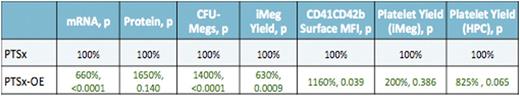
Friend leukemia integration 1 (FLI1) is a critical transcription factor responsible for terminal megakaryocyte differentiation. This transcription factor is amongst the genes missing in the inherited disorder Jacobsen syndrome, resulting from a hemizygous deletion on chromosome 11q. The deletion causes dysmegakaryopoiesis and macrothrombocytopenia termed Paris Trousseau syndrome (PTSx). Also, FLI1 mutations in its DNA-binding domain region results in thrombocytopenia in affected patients. We examined induced pluripotent stem cell- (iPSC) derived megakaryocytes (iMegs) to determine if the platelet disorder observed in PTSx could be replicated and if varied levels of Fli1 expression affected megakaryopoiesis and thrombopoiesis. Beginning with a normal control (WT) iPSC line, genome editing was performed to generate three lines with: 1) one copy of FLI1 disrupted (FLI1+/-), 2) hemizygous transgene expression of Fli1 in the adeno-associated virus site 1 (AAVS1) safe harbor locus using a megakaryocyte-specific GP1balpha promoter (WT-overexpressing line, WT-OE), and 3) homozygous transgene expression in AAVS1 (WT-OE2). Additionally, we established an iPSC line from a PTSx patient and edited this line for a similar hemizygous transgene expression of FLI1 (PTSx-OE). Data described here are summarized in the tables below. We confirm our genome editing strategies by examining mRNA and protein levels of iMegs and found WT-OE and WT-OE2 iMegs have ~3X higher mRNA and ~7X higher protein levels than WT iMegs. FLI1+/- and PTSx iMegs both have lower mRNA and ~0.5X the protein levels of WT iMegs, while PTSx-OE iMegs had comparable levels of mRNA and protein as WT-OE iMegs. Megacult colony assays showed WT-OE and WT-OE2 yielded more CFU-Megs than WT HPCs (p²0.01, p²0.05, respectively). PTSx and FLI1+/- had much less CFU-Megs compared to PTSx-OE and WT (p²0.0001). After growth in liquid culture, WT-OE and -OE2 lines had an increase in iMeg numbers (p=0.29, p²0.01) while FLI1+/- and PTSx iMeg numbers were 43% and 10% that of the WT control (p²0.01, p²0.001). PTSx-OE iMeg numbers were comparable to WT. Surface marker CD41 and CD42b levels were increased compared to WT iMegs in the WT-OE and -OE2 iMegs (p=0.25, p²0.05) and less on FLI1+/- and PTSx iMegs (p²0.01, p²0.05). PTSx-OE iMegs were normal compared to WT, but p²0.05 vs. PTSx. Infused CD41+CD42b+ iMegs into NSG mice showed a trend toward same yield of platelets in the OE lines and lower yield in FLI1+/- and PTSx lines compared to WT. However, when calculations were made from HPCs rather than iMegs infused, the FLI1+/- and PTSx iMegs generated significantly less number of released platelets (p²0.05, p²0.01). The half-life of WT-OE and -OE2 released platelets were increased at 4 and 10 hours compared to WT, whereas FLI1+/- and PTSx released platelets have lower half-lives of 2 hours and 0.5 hours, respectively. These decreased platelet half-lives are due to the majority of platelets being defective and cleared at a higher rate. This was corrected to WT in the PTSx-OE-released platelets. Both in vitro iMegs and in vivo released platelets within the murine blood were assessed for function. Analysis of activation by thrombin was performed via FACS for PAC-1 binding and cell surface P-selectin levels, which are both indicators of platelet activation. Initial studies show no difference between WT and the WT-OE and WT-OE2 lines, but FLI1+/- platelet activation was slightly impaired. In summary, we show that studies of iMegs with decreased Fli1 levels replicate many of the clinical features previously described: less iMegs, lower CD41 and CD42b density with less platelets released while having shorter half-lives. On the other hand, increased Fli1 levels resulted in higher number of iMegs with more surface antigen density and released platelets with increased half-lives. Based on overexpression studies of other transcription factors during megakaryopoiesis, such as GATA1, the expectation would have been that the excess of Fli1 in the OE lines would have also resulted in defects in megakaryopoiesis and thrombopoiesis, but instead improvements were seen. The basis for this difference when overexpressing different transcriptional factors needs further analysis. That high Fli1 levels enhance iMeg yield with maintained numbers and function of released platelets may be of value for generating platelets for clinical use from in vitro grown Megs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal