Abstract
Introduction: Patients with cirrhosis have historically been excluded from clinical trials of anticoagulant therapies due to concerns about safety. While erroneously thought to be "auto-anticoagulated," patients with cirrhosis may actually be at increased risk for venous thromboemboli (VTE) due to an imbalance in pro coagulant and anticoagulant factors and do require therapeutic anticoagulation when thrombotic events occur. Traditionally low molecular weight heparins, and less commonly warfarin, have been the anticoagulants of choice in cirrhotic patients, though there are challenges with their use due to difficulty with access, administration and blood monitoring. Direct Oral Anticoagulants (DOACs) are increasingly replacing warfarin for the treatment of VTE and for stroke prevention in non-valvar atrial fibrillation. The administration and standardized dosing make DOACs an attractive alternative for safe and effective therapeutic anticoagulation but very little evidence exists for their use in patients with cirrhosis.
Methods: We designed a retrospective analysis of all patients with cirrhosis at the University of Virginia who were treated with a DOAC between January 2012 and May 2016 for all indications. All patients were required to be treated with a DOAC with documented follow up for assessment of adverse events. Medical charts were reviewed for age, gender, weight, cirrhosis etiology, DOAC, dose, indication and length of treatment, Model for End-Stage Liver Disease (MELD) and Child-Turcotte-Pugh (CTP) scores, bleeding events and appearance of thrombus on repeat imaging. All bleeding events were recorded and graded according the Common Toxicity Criteria for Adverse Events, v4.0. Major bleeding events were reviewed by all investigators.
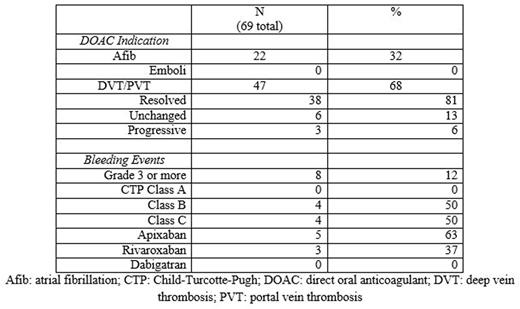
Results: Eighty patients were identified, with 11 patients excluded after not starting a DOAC or being mislabeled as cirrhosis, leaving 69 patients for analysis. Median age was 73 years old (range 44-92) with 77% male. The most common etiologies of cirrhosis were NASH (66%) and HCV (22%). Thirty seven patients (54%) were treated with apixaban, 25 (36%) were treated with rivaroxaban and 7 (10%) were treated with dabigatran. Median length of time on anticoagulation was 6 months. Sixty-eight percent were treated for non-tumoral PVT or DVT while 32% were treated for stroke prevention in atrial fibrillation. Most patients had well compensated cirrhosis, but several had advanced cirrhosis (CTP class A/B/C: 33/26/10). Fourteen percent had an underlying malignancy, most commonly hepatocellular carcinoma, and 52% had esophageal varices. Of those treated for atrial fibrillation, none developed a clot while on a DOAC. Of those treated for VTE, 81% had resolution, 13% unchanged and 6% progression of their clot on repeat imaging. No patient developed drug induced liver injury or discontinued anticoagulation due to liver injury. Sixteen patients (23%) suffered a bleeding event, mostly epistaxis and easy bruising. Major bleeding, defined as grade 3 or higher, occurred in 12% of patients. Most major bleeding was gastrointestinal bleeding; with most but not all requiring endoscopy and transfusions. One subdural hematoma occurred. No variceal bleeding occurred, despite that majority of patients having esophageal varices identified on endoscopy. Of those with major bleeding, all were CTP class B or C with an average MELD of 22, compared to an average MELD of 14 in patients without major bleeding. All patients with major bleeding were treated with either apixaban or rivaroxaban.
Conclusion: Similar to other anticoagulants, DOACs should be used with caution in patients with cirrhosis, particularly CTP class B or C. In this case series, the largest to our knowledge, direct oral anticoagulants in patients with cirrhosis compare favorably to historical controls (Delgado, Clin Gastroenterol Hepatol. 2012 Jul;10(7):776-83) with lower incidence of major bleeding and increased clot resolution. Further prospective studies are needed in this at risk and under studied patient population.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal