Abstract
Introduction
Nephrotic syndrome (NS) is characterized by massive proteinuria (secondary to podocyte injury), hypoalbuminemia, and edema. Importantly, NS is associated with a complex acquired hypercoagulopathy and a high prevalence (~25%) of life-threatening thrombotic complications. We have recently demonstrated that NS disease severity is directly proportional to hypercoagulability,in two well-established animal models of NS (puromycin aminonucleoside (PAN) and Adriamycin (ADR) rats). Furthermore, thromboelastometry studies by our group and others have suggested a resistance to fibrinolysis in both rats and humans with NS. Increasing evidence suggests that myriad abnormalities of the hemostatic system may be implicated in the pathogenesis of altered fibrin clot formation during NS; however the relationship between hypofibrinolysis and NS disease severity requires further validation and the mechanisms underlying the hypofibrinolytic defect remain unknown. Thus, the aim of the present study was to delineate the relationship between disease severity (proteinuria and hypoalbuminemia) and hypofibrinolysis using two rodent models of NS: a transgenic rat expressing the human diphtheria toxin receptor (hDTR) in a podocyte-specific manner and the well-established PAN rat model. We hypothesized that hypofibrinolysis is directly proportional to NS disease severity.
Methods
Using the hDTR rat, we compared markers of global hemostasis (ROTEM) and fibrinolysis to disease severity. A range of severity was induced by a single I.P. injection of diphtheria toxin (0, 25, 50 & 75 ng/kg; n= 7-8/group). On day 10 post-injection, morning spot urines were collected and analyzed for protein:creatinine ratio. Rats were then anesthetized and venous blood (IVC) was collected into 0.32% NaCitrate/1.45 µM Corn Trypsin Inhibitor [final concentrations] and immediately analyzed with ROTEM (whole blood; intem) before being spun down to platelet poor plasma (PPP). Plasma clot lysis assay (CLA) was performed using urokinase (50 IU). Plasmin generation was measured using a fluorogenic substrate in plasma with addition of 10 nM tissue plasminogen activator (tPA).
Results
Hypercoagulopathyin the hDTR NS model: Disease severity (proteinuria and hypoalbuminemia) was significantly correlated with endogenous thrombin potential, peak thrombin, and hypercoagulopathic ROTEM parameters (clot formation time, intermediate firmness (amplitude at 10 & 20 min), and maximum clot firmness).
Hypofibrinolysis: The amount of lysis at 60 min (LI60) on ROTEM was significantly correlated with proteinuria in both the DTR and PAN models (R2=0.167; P=0.015 & R2=0.740; P<0.001, respectively), suggesting that NS thrombi are resistant to fibrinolysis in a manner that is proportional to disease severity. These findings were confirmed with the CLA, such that plasma clot lysis was significantly negatively correlated with proteinuria (R2=0.196; P=0.007 & R2=0.214; P=0.010) and significantly positively correlated with hypoalbuminemia (R2=0.310; P<0.001 & R2=0.240; P=0.006), in the DTR & PAN models, respectively.
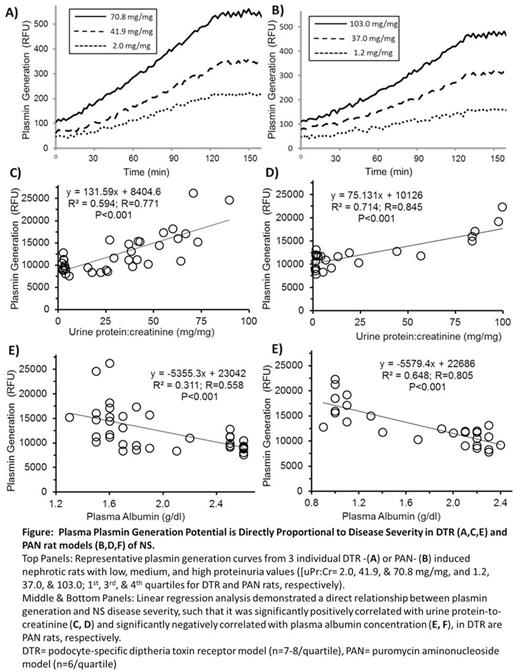
Plasmin Generation Assay: Plasmin generation increased with disease severity, such that it was highly correlated with proteinuria and hypoalbuminemia in both models (P<0.001; Figure). Interestingly, there was no correlation between plasmin generation and plasma clot lysis. (P=0.101 & P=0.126 in DTR & PAN rats, respectively).
Conclusions
NS disease severity is directly proportional to hypercoagulability in a podocyte-specific rodent model of NS; thus confirming our previously published findings in a podocyte-specific experimental model of NS. Moreover, this hypercoagulopathy leads to formation of a clot that is resistant to fibrinolysis. Paradoxically, the increased plasmin generation implies that the fibrinolytic system is also hyperactive in NS. Thus, it must be concluded that the clot is resistant, even to this increased plasmin that is generated. Future studies will explore the mechanisms underlying and in vivo significance of this fibrinolytic resistance.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal