Abstract
Background: Human neutrophil peptides (HNPs) are small cationic proteins primarily released from activated and degranulated neutrophils. HNPs have antimicrobial activity against diverse bacteria, viruses, fungi, and parasites. Additionally, HNPs exhibit prothrombotic properties by enhancing platelet aggregation and fibrin formation and by inhibiting proteolytic cleavage of von Willebrand factor (VWF) by ADAMTS13. However, the role of HNPs in thrombus formation under more physiological conditions (i.e. under flow) has not been determined.
Objective: To investigate the effects of HNPs on platelet adhesion/aggregation on VWF/collagen surfaces under arterial shears.
Design/Method: Whole blood was obtained from C57/BL6 wild type and Adamts13-/-mice, anticoagulated with a potent thrombin inhibitor D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK) and prostaglandin-E1 (PGE1), and platelets were labeled with FITC anti-mouse CD41 IgG. After incubation with varying concentrations of native HNPs and synthetic partially reduced HNP1 (sHNP1) for 30 minutes, the whole blood samples were perfused through a fibrillar collagen-coated surface in a microfluidic system at 100 dyne/cm² for 180 seconds. The rate and extent of accumulation of fluorescein-labeled platelets were determined under an inverted fluorescent microscope at 4-second intervals. The images were analyzed off-line with Montage to evaluate the area of platelet coverage over time. This process was repeated with the addition of N-ethylmaleimide (NEM) alone or NEM-treated sHNP1 to the samples to probe the effect of free cysteine residues. In addition, samples of native HNPs and sHNP1 incubated with NEM were analyzed via LC-mass spectrometry for NEM incorporation.
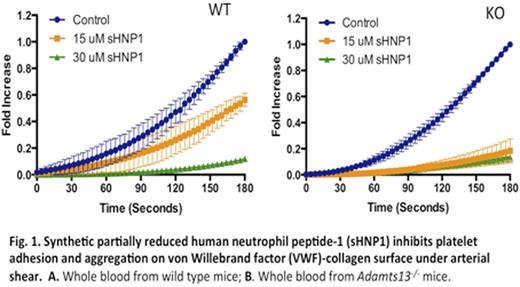
Results: Purified native HNPs at final concentrations of 15 μM and 30 μM exhibited no or little effect on the adhesion and aggregation of murine platelets on VWF/collagen surfaces under arterial shears (100 dyne/cm2). Surprisingly, sHNP1 at the same concentrations (15 and 30 μM) dramatically reduced the rate and surface coverage of platelets from WT (Fig. 1A) and, more profoundly, from Adamts13-/- mice (Fig. 1B) on VWF/collagen surfaces under the same conditions. This inhibitory activity of sHNP1 was abolished upon pretreatment with NEM, which reacts with free thiols (-SH) (not shown). Aliphatic HNP1 with all 6 cysteine residues chemically modified also did not inhibit the adhesion and aggregation of murine platelets on VWF/collagen surfaces under shear (not shown). Analysis of samples by LC-mass spectrometry confirmed the NEM-labeling of free thiols present in sHNP1, but not in native HNPs.
Conclusion: These results suggest that high concentrations of locally released native HNPs may be required to inhibit ADAMTS13 activity in vivo. However, the findings from this study indicate that HNPs differentially affect thrombus formation depending on how its redox state is modified by its biological milieu. Somewhat unexpectedly, synthetic and partially reduced HNP1 may be a potent antithrombotic agent by reducing platelet interactions with VWF under arterial shear via a disulfide bond reduction mechanism.
Zheng:Alexion: Research Funding; Ablynx: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal