Abstract
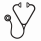
A tightly regulated network of intrinsic and extrinsic signaling pathways exists to preserve HSC pool size and function. This is particularly relevant during hematopoietic injuries when dormant HSCs transiently start to proliferate to replenish blood cells; as unbalanced HSC proliferation can lead to stem cell exhaustion, long-term myelosuppression and death. Although there has been growing interest in how circulating sex hormones influence HSC function (Nakada et al., 2014; Sanchez-Aguilera et al., 2014), this pathway remains poorly understood. Here we describe a heretofore unknown role for the upstream hormone regulator, luteinizing hormone (LH), in regulating HSC biology.
We found that both human and mouse HSCs highly expressed the LH receptor, and its expression was decreased or nearly absent in downstream progenitors (Figure 1a). LH significantly promoted HSC colony forming potential in cobblestone area-forming cell and colony-forming cell assays that, together with expression of the receptor, suggested that LH increased HSC expansion in vitro by acting directly on the most primitive HSCs.
To investigate whether LH levels could impact on HSC pool size during hematopoietic stress in vivo, we challenged mice using models that force HSCs out of their quiescent status, Poly I:C and sub-lethal dose of total body irradiation (SL-TBI, 550cGy). We found that ablation of LH production using a luteinizing hormone-releasing hormone-antagonist (LHRH-Ant) retained significantly more HSCs in G0 in both models (Figure 1b,c).
Previous reports have shown that induction of HSC quiescence after high-dose irradiation correlates with increased hematopoietic recovery and enhanced mouse survival (Chen et al., 2008; Himburg et al., 2014; Johnson et al., 2010). Given its effectiveness in promoting HSC quiescence and the fact that LHRH-Ant are widely available and clinically approved, we hypothesized that LHRH-Ant could represent a rational non-cellular medical countermeasure for mitigating radiation injury and promoting hematopoietic regeneration when administered after hematopoietic insult. To test this hypothesis, we used a lethal TBI (L-TBI) dose of 840cGy that mediated lethality in more than 90% of B6 male mice. We found that pharmacological inhibition of LH using LHRH-Ant 24h after L-TBI spared the most primitive long term HSCs (Figure 1d) thus promoting hematopoietic recovery and mouse survival (Figure 1e). Consistent with our original hypothesis we also found a significantly higher proportion of Ki-67− quiescent HSCs in the LHRH-Ant-treated group with fewer proliferative HSCs compared to controls. Given the wide-ranging hormonal changes induced by LHRH suppression and the previously reported effects mediated by sex steroid ablation on hematopoietic stem/progenitor cell (HSPC) compartment (Khong et al., 2015), we next evaluated whether the LHRH-Ant effects on mouse survival after L-TBI were independent from the suppression of the downstream sex steroids. Administration of LHRH-Ant improved survival rates in surgically castrated mice following radiation injury, while surgical castration alone did not, indicating that the regenerative effects were independent from downstream sex steroids. To confirm whether the protective effects of LHRH-Ant treatment depended on suppression of LH, we administered the LH receptor agonist human chorionic gonadotropin (hCG) to LHRH-Ant treated mice that had been given L-TBI one-day prior. Consistent with our hypothesis, administration of hCG abrogated the beneficial effects of LHRH-Ant on survival after radiation injury.
Taken together our studies showed that HSCs are a physiological target of LH, which promotes their proliferation. Furthermore, pharmacological inhibition of LH signaling using a single dose of an LHRH-Ant represents a rational and feasible approach to preserve the HSC pool after high dose radiation, thereby mitigating acute hematopoietic radiation syndrome.
Van Den Brink:Seres: Research Funding; Novartis: Consultancy; Regeneron: Consultancy; Flagship Ventures: Consultancy; Boehringer Ingelheim: Consultancy; Merck: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal