Abstract
Elevated systemic blood pressure and chronic kidney disease are commonly observed in people with sickle cell disease (SCD) and are independent predictors for increased morbidity and early mortality. Based on limited studies with small samples sizes and short durations of follow up, angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) are used to treat SCD patients with elevated urine albumin concentration expressed as albumin-to-creatinine ratio. We conducted a retrospective study to evaluate the effects of ACE-inhibitors and ARBs on urine albumin concentration and systemic blood pressure and to record potential toxicities of these agents in SCD patients receiving their care at the University of Illinois at Chicago. Comparisons of variables from pre-therapy to therapy of 1 year ± 6 months duration were conducted using the paired t-test or repeated measures ANOVA.
Between 11/1/2001 and 11/1/2015, 64 patients were prescribed an ACE-inhibitor (n=43) or ARB (n=21) by their treating physician. The median age at the time of prescription was 37 years old (interquartile range 29-49 years), 40% were female, and the majority had hemoglobin SS disease (Hb SS: n=54, Hb SC n=9, Hb Sβ+-thalassemia, n=1).
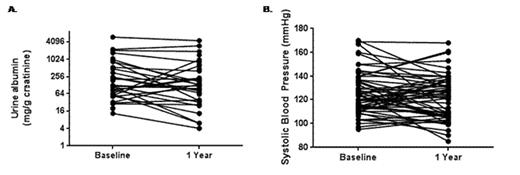
The urine albumin concentration was available both pre-treatment and after 1 year of treatment with ACE-inhibitor or ARB therapy in 29 patients. Trends for decreases in urine albumin concentration (baseline 166±37 mg/g creatinine vs. 1-year 104±28 mg/g creatinine; one-sided P=0.04) (Figure 1A) and systolic blood pressure (baseline 126±2 mmHg vs. 1-year 121±2 mmHg; one-sided P=0.03) (Figure 1B) were observed. The starting dose of the ACE-inhibitor or ARB was a significant predictor of the decrease in urine albumin (P=0.02) and this association persisted after adjusting for hemoglobin SS genotype (P=0.03) or age (P=0.02). Trends for associations between the starting dose and a 50% decline in urine albumin (P=0.09) were also observed. No significant urine albumin changes based on ACE-inhibitor versus ARB therapy or the concomitant use of hydroxyurea or other anti-hypertensive medications were observed.
Assessment for toxicity in all 64 patients during the observation period revealed the following. Two patients developed hyperkalemia and 1 developed worsening kidney disease requiring the ACE-inhibitor or ARB therapy to be discontinued. In those that continued therapy, other potential toxicities included trends for a reduction in mean (±SEM) estimated glomerular filtration rate (baseline 126±8 mL/min/1.73m2 vs. 1-year 115±9 mL/min/1.73m2; P=0.1), a decline in hemoglobin concentration (9.1±0.2 g/dL vs. 8.8±0.2 g/dL; P=0.08), and an increase in serum potassium concentration (4.3±0.05 mEq/L vs. 4.4±0.07mEq/L, P=0.04).
In conclusion, ACE-inhibitors or ARBs were associated with trends for reduction in urine albumin and systolic blood pressure at 1 year of follow up and the initiating dose may influence the urine albumin response. Toxicities that need to be closely monitored include hyperkalemia and worsening kidney function. Our findings highlight the need for a prospective, randomized study to evaluate the starting dose, efficacy and safety of ACE-inhibitor and ARB therapy in SCD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal