Abstract
Background: Novel molecular and immunophenotypic markers in Diffuse Large B-Cell Lymphoma (DLBCL) are of interest for potential therapeutic guidance in addition to prognostication. The cell-surface marker CD30 is expressed by several types of non-Hodgkin lymphomas, including DLBCL. A piqued interest to explore the prevalence of this marker in different DLBCLs is driven by the availability of an FDA-approved anti-CD30 monoclonal antibody; brentuximab. We identified the prevalence of CD30 in de novo and relapsed DLBCL in a single medical center and investigated its relation with the clinical and biochemical characteristics of our patients as well as their survival data.
Methods: This is an IRB-approved retrospective cohort study of consecutive patients diagnosed with de novo DLBCL at the Washington, DC Veterans Affairs Medical Center between 1995 and 2014. Patients with other lymphoma types or unavailable archived pathology specimens for testing were excluded. Additional pathology review of all specimens diagnosed as DLBCL was undertaken by our pathologist and questionable cases were excluded. Clinical and laboratory data including age, rituximab use, chemotherapy regimen, hepatitis C (HCV) and Human Immunodeficiency Virus (HIV) infection, organ involvement, stage, and prognostic scores were collected both at diagnosis as well as at relapse. Immunohistochemistry (IHC) was performed on formalin-fixed, paraffin-embedded tissue sections from all patients to examine the following expressions: CD3, CD5, CD10, CD20, CD30, bcl-2, bcl-6, MUM-1, and MYC. CD30 expression was assessed; absence of tumor cell staining interpreted as 0% expression, and the percentage of positive tumor cells reported. CD30-positive and CD30-negative clinical and pathologic variables and overall survival (OS) were analyzed. Distributions were estimated using Kaplan-Meier analysis, and log-rank test was used to assess the survival differences. Statistical analysis was performed using SPSS software version 20.0.
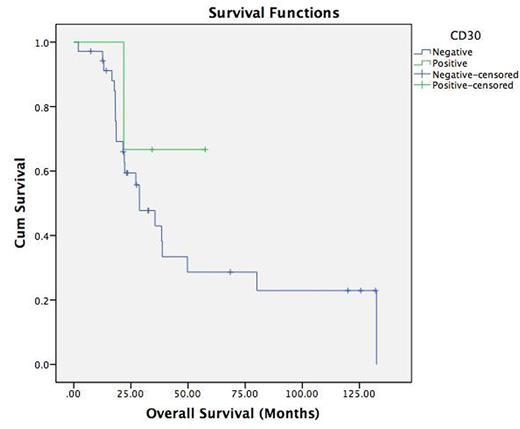
Results: A total of 69 subjects with DLBCL were identified and 42 met the inclusion criteria with available archived pathology blocks for review and testing. Median age at diagnosis was 66 years. Only one female was in the study. Nodal and extranodal disease was 29.2% and 70.8%, respectively at diagnosis while only 2.4% of patients had only nodal disease at relapse. Localized (I&II) and advanced (III &IV) disease stages were noted in 34.3% and 65.7%, respectively and only 6.8% of patients had localized disease at relapse. Viral serology revealed HIV in 7.1% and HCV in 9%. LDH at diagnosis was on average 386.7 ± 270.9 IU/L. Chemotherapy regimens used included CHOP±R, EPOCH±R, ProMACE CytaBOM, and rituximab/gemcitabine/oxaliplatin/dexamethasone with the majority receiving R-EPOCH as first line chemotherapy (33.3%). Prevalence of positive IHC markers were as follows; CD3 (2.4 %), CD5 (7.2%), CD10 (28.6%), CD20 (100%), CD30 (7.1%), bcl-2 (71.4%), bcl-6 (71.4%), MUM-1(15%), c-MYC (38.1%) and P53 (16.5%). None of the CD30-positive patients had c-MYC rearrangement. Median overall survival (OS) was 23.3 months for the whole cohort. It was noted to be higher in the CD30-positive group compared to the CD30-negative group (28.7 and 34 months, respectively). The difference in OS was not statistically significant, however. CD30 status and stage at presentation was not analyzed given the low prevalence of CD30 in our cohort.
Conclusions: In conclusion, our study identified 7.1% CD30-positive patients with de novo or relapsed DLBCL, all of which had greater than 50% CD30 expression. While rate of expression does not correlate with response to brentuximab, 7.1% in our cohort could potentially benefit from brentuximab. Our cohort had a low prevalence of CD30 expression. However, higher OS for the CD30 positive cohort was noted, despite not being significant. The low prevalence of CD30 expression in our cohort maybe explained by the fact that they had a higher prevalence of MYC expression suggesting a possible mutually exclusive relationship. A larger cohort is necessary to assess if this relationship is reproducible and statistically significant. Possible implications of such a mutually exclusive relationship includes the option to omit FISH for MYC rearrangement testing in cases with high CD30 expression.
Kaplan-Meier curve for the overall survival stratified according to CD30 status (p=0.389).
Kaplan-Meier curve for the overall survival stratified according to CD30 status (p=0.389).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal