Abstract
INTRODUCTION: Non-Hodgkin's lymphoma is a heterogeneous group of diseases originating in the lymphoid system. The most common subtype, constituting up to 40% of all cases globally, is diffuse large B cell lymphoma (DLBCL).1 Approximately one-third of DLBCL patients treated with standard therapy (since 1998, a combination of chemotherapy plus immunotherapy with the CD20-directed humanized monoclonal antibody rituximab [RCHOP]) at diagnosis will develop relapsed or refractory (R/R) disease.1 Relapsed or refractory disease is defined as those whose disease does not respond to therapy (refractory) or whose disease returns after achieving remission (relapse), requiring secondary therapies. In this systematic review we examine outcomes associated with therapies used to treat R/R DLBCL including autologous (auto) stem cell transplant (SCT) and allogeneic SCT (alloSCT), plus various chemotherapy regimens in patients not eligible for SCT.
METHODS: Literature databases (Medline and EMBASE) were searched for the past five years (from January 1, 2012-May 11, 2016) to identify studies reporting treatment outcomes in R/R DLBCL (including patients who had failed at least one prior treatment line). The searches applied terms to targeted publications on relapsed/refractory DLBCL and were limited to studies conducted in humans and articles published in English, including an abstract. To ensure the latest information available was included, the database searches were supplemented by reviewing American Society of Hematology (ASH) conference abstracts from the last two years. Only studies clearly (as denoted in the abstract) enrolling R/R patients with a sample size of at least 50 were included to ensure the evidence collected was well powered to detect meaningful findings. Furthermore, the review was limited by geographic location to studies primarily conducted in the European Union 5 (EU5), United States (US), and Japan.
RESULTS: Of the 590 abstracts identified through Medline and EMBASE and the 325 ASH abstracts, 21 studies met the inclusion criteria after reviewing the full-text articles. Those studies meeting the criteria clearly presented clinical results for at least 50 patients with R/R DLBCL. The evidence was primarily available in observational (16/21) study designs; most of the data was presented in ASH abstracts (13/21) from the past two years. Of the clinical trials (four Phase II, one Phase II/III), many evaluated chemotherapy in patients ineligible for SCT or high dose salvage regimens. Among the studies included, 8 enrolled patients from the EU, 7 from the US, 4 from Japan, and 2 with sites in both the US and EU. Of the identified studies, there were 7 real-world observational studies evaluating SCT in R/R DLBCL. The majority (71%) investigated alloSCT with reported overall survival (OS) rates ranging from 18-52%. Among the three studies examining autoSCT, OS ranged from 54-71% (Table 1). Only one study (Rigacci, 2012) reported data on response after transplant (overall response [OR] 49%; complete response [CR] 43%).
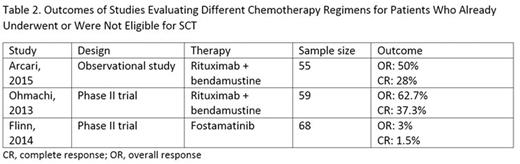
For post-SCT or SCT-ineligible patients, various chemotherapy regimens were used. Three articles were identified examining chemotherapy regimens in this setting, two of which were Phase II clinical trials. OR rates ranged widely from 3% to 62.7% for trials investigating fostamatinib and rituximab + bendamustine, respectively (Table 2). Limited data on survival were available, with only Arcari, 2015 reporting median survival of 10.8 months for patients receiving rituximab + bendamustine.
CONCLUSIONS: In the past five years, only limited clinical evidence has been reported specifically for patients with R/R DLBCL. Among studies examining DLBCL, treatment courses ranged from SCT to combination chemotherapy. Survival outcomes were more often reported in studies evaluating patients treated with SCT (auto or allo) than in studies evaluating those post-SCT or SCT-ineligible. Rates of survival at three or four years varied widely from 18% to nearly two-thirds to three-quarters of patients treated with SCT; survival was higher in patients treated with autoSCT. Response rates in those ineligible for SCT were also highly variable. Studies examining SCT that were included in the review had only limited information on response.
1 World Cancer Report 2014. International Agency for Research on Cancer. (2014) Accessed July 2014.
Galaznik:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment, Equity Ownership. Bell:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment, Equity Ownership. Hoog:Evidera Inc.: Employment. Stokes:Evidera Inc.: Employment. Steenrod:Evidera Inc.: Employment. Knopf:Sutter Health Care: Employment; Exeltis: Speakers Bureau; Evidera Inc.: Consultancy. Seal:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment, Equity Ownership. Shou:Millennium Pharmaceuticals Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal