Abstract
Introduction: The standard of care for treatment of cancer-related venous thromboembolism (VTE) is a low molecular heparin (LMWH). In our previous claimsbased analysis, we showed that besides LMWH oral anticoagulants, warafrin and rivaroxaban are widely prescribed in clinical practice (Khorana, 2015). The objective of this study is to compare VTE-related healthcare resource utilization and costs of cancer patients treated with anticoagulant therapies.
Methods: Medical and pharmacy claims from the Humana Database between 1/1/2013 and 05/31/2015 were analyzed. Newly diagnosed cancer patients with a first VTE diagnosis occurring after their first cancer diagnosis and with ≥1 dispensing of an anticoagulant agent within 7 days after their VTE diagnosis, were selected. Based on the first anticoagulant agent received, patients were classified into one of the following cohorts: LMWH, warfarin, and rivaroxaban. Inverse probability of treatment weights based on propensity score was used to adjust for differences between treatment cohorts. Baseline characteristics were evaluated during the 6 month period prior to the index VTE. VTE-related resource utilization and costs were identified with a primary or secondary diagnosis of deep vein thrombosis or pulmonary embolism and were evaluated for the entire follow up period, starting from the initiation of the anticoagulant therapy until the earliest either end of enrollment or end of data availability (05/31/2015). Resource utilization components included: number of hospitalizations, hospitalization days, emergency room (ER) visits, and outpatient visits. Comparisons between the different treatment cohorts were performed using rate ratios (RR) and statistical differences between groups as well as 95% confidence intervals [95% CI] were calculated using Poisson regression models. Healthcare costs were evaluated in per-patient-per-year (PPPY) and compared using mean cost difference.
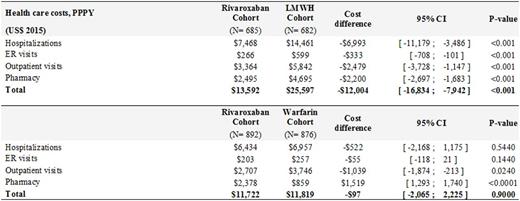
Results: A total of 2,428 patients (LMWH: n=660; warfarin; n=1,061; rivaroxaban: n=707) were included. Baseline demographic and clinical characteristics were well balanced across the treatment cohorts including hospitalizations, emergency room (ER) visits, and outpatient visits. Compared to patients treated with LMWH, patients treated with rivaroxaban had significantly fewer VTE-related hospitalizations, hospitalization days, ER visits, and outpatient visits (Figure 1). This resulted in significantly higher VTE-related health care cost difference of $12,000 for LMWH relative to rivaroxaban treatment cohort (Table 1).
Patients treated with rivaroxaban had significantly lower VTE-related resource utilization compared to patients treated with warfarin (Figure 1). The total VTE-related costs were however similar between two cohorts (Table 1). The higher drug costs ($1,519) were offset by significantly lower outpatient (-$1,039) and hospitalization costs (-$522) in rivaroxaban relative to warfarin cohort.
Conclusion: Healthcare resource use and costs associated with VTE treatment in cancer patients are the highest with LMWH relative to warfarin and rivaroxaban treatments. Healthcare resources use and costs are significantly higher in warfarin relative to rivaroxaban cohort but these resource costs were offset by higher drug costs of rivaroxaban. These results are in line with previous study in cancer patients that found fewer re-hospitalizations related to VTE recurrences in patients treated with rivaroxaban compared to patient treated with LMWH or warfarin (Streiff, 2016).
VTE-Related Healthcare Resource Utilization
VTE-Related Health Care Costs, PPPY
Streiff:Roche: Research Funding; Portola: Research Funding; Janssen: Consultancy, Research Funding; CSL Behring: Consultancy, Research Funding. Milentijevic:Janssen Scientific Affairs: Employment, Equity Ownership. McCrae:Janssen: Membership on an entity's Board of Directors or advisory committees. Fortier:Janssen Pharmaceuticals: Research Funding. Laliberté:Janssen Scientific Affairs: Research Funding. Lefebvre:Janssen Scientific Affairs: Research Funding. Schein:Johnson & Johnson: Employment, Equity Ownership, Other: Own in excess of $10,000 of J&J stock. Khorana:Sanofi: Consultancy, Honoraria; Leo: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria; Halozyme: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Janssen Scientific Affairs, LLC: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal