Abstract
Despite significant progress in elucidating the predictive value of somatic mutations in AML following conventional chemotherapy, the implications of extended genomic profiling in patients undergoing allogeneic hematopoietic stem cell transplant (allo-HSCT) are poorly understood. Moreover, little work has been performed correlating somatic mutations identified on an extended gene panel with AML relapse and survival following allo-HSCT, and comparison of the prognostic significance of somatic mutations, transplant conditioning intensity, stem cell source, AML disease types and cytogenetics in an unselected cohort of AML patients undergoing allo-HSCT has not been described.
We performed mutation profiling on diagnostic bone marrow samples from 153 patients with AML who underwent allo-HSCT. Median age was 58 years (19-78), and median follow-up after allo-HSCT was 8 months (1-33). Three sequencing platforms were used (Foundation Medicine Hematology NGS panel, N=32; Institutional 28 gene myeloid panel, N=51; 6 gene PCR panel, N=70). Hazard ratios (HR) for relapse and OS were calculated for genes mutated in 5 or more patients (IDH 1/2, JAK2, DNMT3A, FLT3 ITD & TKD, NPM1, TET2, RUNX1, ASXL1 and TP53). Secondary AML was defined as AML arising from a prior myeloid disorder or therapy related AML. Categorical variables were compared with Fischers exact test and continuous variables using Kruskal-Wallis test. Cox regression estimated hazard ratios (HR) for factors associated with relapse and OS post-allo-HSCT. Significant factors were included in multivariate analysis.
Factors associated with significantly reduced OS following allo-HSCT included blasts over 5% prior to allo-HSCT, secondary AML, therapy-related AML, monosomal karyotype, mutant RAS and mutant TP53. Blasts over 5% before allo-HSCT, complex or monosomal karyotype and TP53 mutation were associated with risk of relapse (Table 1). No other somatic mutations predicted relapse. Median OS from diagnosis for TP53-mutated AML patients undergoing allo-HSCT was 6 months (Fig 1). Allo-HSCT characteristics were not associated with relapse or OS in this cohort (Table 1).
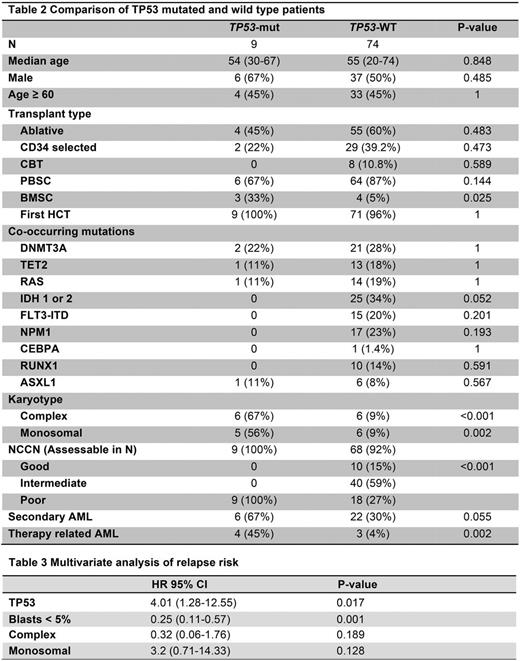
Given its association with OS and relapse, we focused subsequent analyses on patients with mutant TP53. No significant difference in conditioning or donor source was noted between TP53-mutated and wild-type patients. Mutations in TP53 were associated with NCCN poor risk AML, complex karyotype and monosomal karyotype (Table 2). Three patients (33%) with TP53 mutations did not have a complex or monosomal karyotype but did have either MLL rearrangement (N=1) or adverse cytogenetics with del(5q) (N=2). While the number of patients with TP53 mutations was small in this unselected cohort (9/83, 11%), multivariate analysis found that mutations in TP53 and presence of over 5% blasts before allo-HSCT were both predictive of relapse after transplant (table 3).
In the TP53-mutant group, 4/9 patients relapsed and 5/9 patients died. 3 died due to non-relapse mortality, and 2 died due to AML. 2 relapsed patients remained alive at last follow-up; one received azacitidine/DLI and attained a flow-cytometry-based MRD-positive CR before progressing, and the other had progressive disease following Decitabine. 3/4 relapsed patients had sequencing at the time of relapse. TP53 mutations were identified at relapse in all 3 patients, indicating that the TP53 mutated clone was involved in relapse.
In summary, we found that TP53 mutations and pre-transplant bone marrow blasts were associated with relapse after allo-HSCT in this unselected cohort of AML patients. TP53 mutations were associated with NCCN high-risk AML, complex and monosomal karyotype and therapy-related AML. We did not find other somatic mutations to be associated with relapse. Further study in a larger cohort is necessary to confirm the prognostic implications of TP53 mutations and to assess the value of other mutations in predicting outcomes after allo-HSCT. The poor outcomes observed for patients with TP53-mutated AML highlight the need to assess TP53 mutation status to identify patients who may benefit from maintenance strategies or other pharmacologic or cellular approaches after transplant to reduce the risk of relapse in these patients.
Levine:Novartis: Consultancy; Qiagen: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal