Abstract
Introduction: Up to 40% of new acute myeloid leukemia (AML) patients fail to achieve complete remission (CR), and a significant number of patients achieving remission eventually relapse. Allogeneic hematopoietic cell transplantation (allo-HCT) remains the best curative option for these patients with a long-term leukemia free survival (LFS) of around 20-30% with the use of intensive myeloablative condition (MAC) transplants. Active disease AML patients otherwise deemed unfit for MAC regimens have limited treatment options. Although reduced intensity condition (RIC) has broadened transplant eligibility in the elderly AML patients particularly in CR, there is a paucity of data on the use RIC allo-HCT in AML patients with active disease. To answer this question, we retrospectively analyzed data from active disease AML patients who underwent RIC allo-HCT and compared their outcomes with contemporaneous patients who underwent MAC allo-HCT at our institution.
Patients and methods: Our cohort included all patients with active disease AML (primary induction failure, relapsed refractory or cytogenetic persistence) at the time of transplantation, who underwent allo-HCT at Washington University Medical Center in St. Louis between January 2006 and June 2015. Patients were classified according to the intensity of conditioning regimens: MAC versus RIC. The primary study endpoints were leukemia free survival (LFS) and overall survival (OS). LFS was defined as the time from the achievement of CR after allo-HCT to the time of relapse, death in remission, or last follow-up. OS was defined as the time from transplantation to the time of death from any cause or last follow-up. The between-group differences in LFS and OS were described using Kaplan-Meier (KM) survival curves and compared by log-rank test. Univariate Cox regression analysis for LFS was performed. All analyses were two-sided, and significance was set at a p-value of 0.05, using SAS 9.4 (SAS Institutes, Cary, NC).
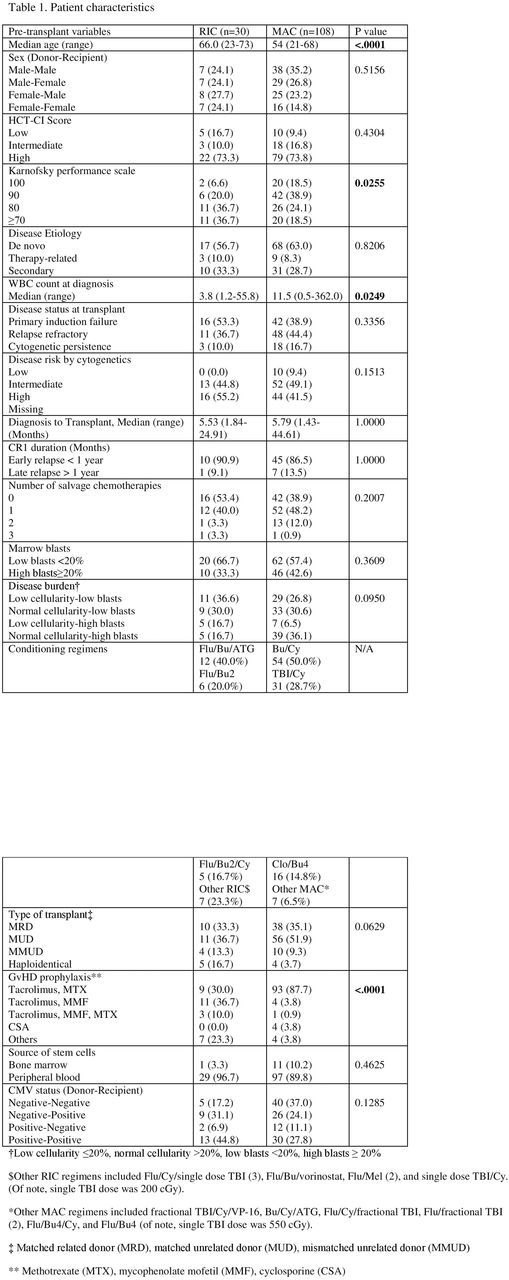
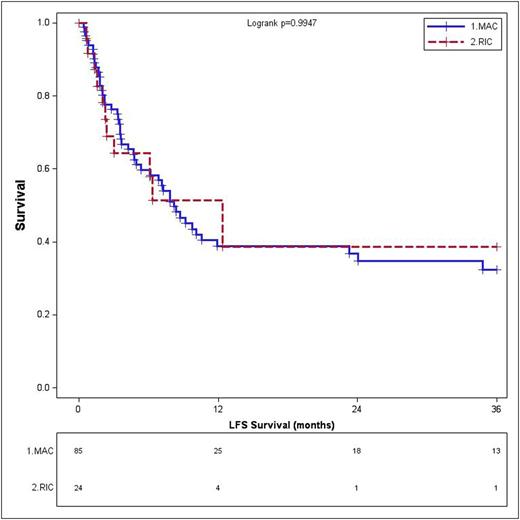
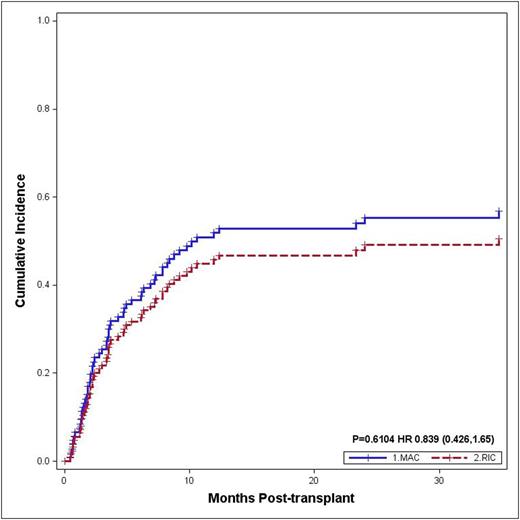
Results: 138 patients were included. 30 patients (21.7%) underwent RIC allo-HCT and 108 (78.3%) underwent MAC allo-HCT. Their baseline characteristics are listed in Table 1. 21 patients (72.4%) in RIC and 77 (81.9%) in MAC achieved CR on day-28 bone marrow biopsy. Notably, there were 16 patients (1 RIC and 15 MAC) who died prematurely without post-transplant evaluation; subsequently they were excluded from LFS, relapse and NRM calculations. There was no difference in the LFS in these two groups; 1-year and 3-year LFS were 40.6% (95% CI 23-70) and 33.1% (95% CI 17-66) in the RIC patients while 40.7% (95% CI 31-54) and 33.2% (95% CI 23-47) in the MAC patients, respectively (p=0.99) (Figure 1A). Similarly the CI of relapse at 1 year and 3 year was not different in the two groups; 45.8% (95% CI 30-71) and 50.5% (95% CI 33-76) in RIC group vs. 51.9% (95% CI 43-63) and 56.8% (95% CI 48-67) in MAC group, respectively (p=0.61) (Figure 1B).
The CI of NRM at 1-year and 3-year was 28.1% (95% CI 16-50) and 32.6% (95% CI 19-56) in RIC group compared to 17.9% (95% CI 11-28) and 21.0% (95% CI 14-31) in the MAC group (p = 0.21). However, OS in both groups remained poor with 1-year and 3-year OS of 25.1% (95% CI 14-44) and 13.2% (95% CI 6-31) in RIC vs. 35.5% (95% CI 28-46) and 22.0% (95% CI 15-32) in MAC, respectively (p = 0.21). On univariate analysis for LFS and relapse, only cGvHD was associated with higher LFS (p<0.01, HR 0.27, 95% HR 0.13-0.52) and lower relapse risk (p= 0.01, HR 0.37, 95% HR 0.15-0.89) in these patients. On multivariate analysis for LFS, only cGvHD was statistically significant (p<0.01, HR 0.32, 95% HR 0.18-0.56). On the other hand, intermediate cytogenetic risk (p=0.01, HR 1.59, 95% HR 1.08-2.34) and cGvHD (p<0.01, HR 0.12, 95% HR 0.05-0.27) were significant for OS.
Conclusion: The use of RIC allo-HCT in active disease AML is associated with LFS and relapse comparable to MAC allo-HCT. However, high relapse rates and NRM, in both groups translated into poor long term OS. Careful selection of patients and early utilization of transplant without subjecting these patients to multiple salvage regimens might help lower NRM rates in future studies. Notably, our study might open a window for future prospective studies aimed at finding improved ways to harness the graft versus leukemia (GvL) effect associated with RIC transplantation in patients who are otherwise not able to tolerate more toxic intensive conditioning regimens.
Relapse
DiPersio:Incyte Corporation: Research Funding. Vij:Karyopharm: Honoraria; Janssen: Honoraria; Bristol-Myers Squibb: Honoraria; Novartis: Honoraria; Amgen: Honoraria, Research Funding; Celgene: Consultancy; Takeda: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal