Abstract
The outcomes after Haploidentical HCT with Fludarabine (Flu)/Cyclophosphamide (Cy)/TBI and PTCY as pioneered by the Hopkins group have been associated with higher risk of relapse and delayed immune reconstitution. Multiple groups have explored the potential benefits of more intensive conditioning regimens with or without TBI. However, none of these regimens to our knowledge has exploited the immunogenic properties of pre-transplant low dose Cy while avoiding the immune-suppressive effect of TBI. Additionally, there is limited knowledge regarding immune reconstitution post haploidentical HCT with the use of mobilized peripheral blood stem cells. Accordingly, we designed a protocol modification of the Hopkins regimen by replacing TBI with Melphalan (Mel) without adding Thiotepa in contrast to the MD Anderson regimen (Ciurea et al). We hypothesized that the immunogenic properties of pre-transplant low dose Cy along with the immunomodulatory effects of Mel would enhance early post-transplant engraftment and early peripheral T cell recovery through enhanced cytokine release and antigen alloreactivity
We examined the clinical outcomes and immune reconstitution recovery of 12 consecutive haploidentical HCT patients with high-risk hematological malignancies. Patient and graft characteristics are as shown in table-1. The conditioning regimen was low dose Cy 14.5 mg/m2 on day -6 & day -5, Flu 30 mg/m2 on days -6 to Days -2 and Mel 70 mg/m2 on day -3 & -2. Graft versus host disease prophylaxis (GVHD) was PTCY 50 mg/Kg on day +3 and day +4 along with Tacrolimus and Mycophenolate starting at day +5 as previously described by the Hopkins group. An immune reconstitution panel of absolute lymphocyte count (ALC), CD3, CD4, CD8, Activated T cell (CD3-HLA Dr + T cells) & NK cell (CD56/16) was performed by 4 color flow-cytometry on days +60, +120 and +180 post HCT. Chimerism studies were performed at day +30 and +100 post HCT by variable number tandem repeat PCR analysis of peripheral blood & bone marrow. All of the patients were treated according to an institutional protocol and records were reviewed retrospectively after IRB approval.
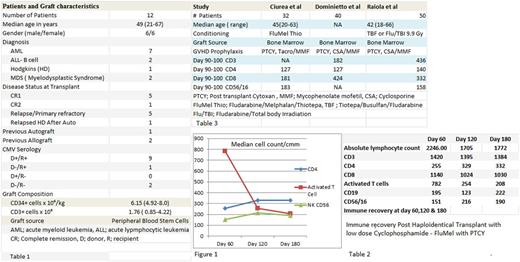
All patients engrafted with a median time to engraftment of 17 days (range 12-23). Chimerism studies revealed enhanced engraftment with 100% donor in bone marrow (unsorted) and peripheral blood (CD3, CD33 & CD 56) at day +30 in all 12 patients. All patients with active disease at time of transplant (5 Pts) achieved complete remission at day +30 evaluation. Post HCT immune recovery is shown in table 2. There was significant early recovery at day + 60 of the median ALC and all T cell subsets. This recovery was most pronounced in activated T cells. While we have observed a progressive reduction in the median number of early activated T cells at day 120 & 180, the number of helper T cells (CD4) and NK cells (CD56/16) did not decline (Figure 1).
With a median duration of follow up of 261 days (range 62-390), the overall survival at day 100 and projected one year survival is 96% and 81% respectively. Only one of 12 patients had relapsed. Acute GVHD grade 2-4 developed in six of 12 patients, two of whom were grade 3-4. Chronic GVHD developed in four patients (1 serositis, 1 pericardial effusion and 2 nephrotic range proteinuria). Cytokine storm developed in 5/12 patients after stem cell infusion and resolved after PTCY. BK cystitis developed in four patients but continuous bladder irrigation was only required in two patients. All cases of BK cystitis were transient and resolved with supportive measures. CMV reactivation occurred in 9/12 patients; no CMV disease or CMV mortality was observed. Aspergillus antigen positivity in serum occurred in 4 /12 but only two developed clinical fungal infection.
Conclusion: In this limited series of patients with high- risk hematological malignancies who underwent haploidentical HCT with low dose Cy/Flu/Mel and PTCY, the regimen was well tolerated and resulted in effective disease control. The regimen has also demonstrated enhanced early engraftment and robust immune recovery in comparison to other studies, table 3. However, it is not clear if this enhanced immune recovery is related to the conditioning regimen modification or the use of mobilized stem cells. The results are intriguing and need further confirmation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal