Abstract
Introduction: IDH2 mutations (mIDH2) are recurrent in ~5% of patients (pts) with MDS and ~15% of pts with acute myeloid leukemia (AML). mIDH2 proteins have neomorphic enzymatic activity and are associated with DNA and histone hypermethylation, altered gene expression, and blocked differentiation of hematopoietic progenitor cells. Enasidenib (AG-221/CC-90007) is a small-molecule allosteric inhibitor of mIDH2 protein that induces hematological responses in pts with mIDH2 AML, including relapsed or refractory (R/R) AML (Stein, Clin Cancer Res, 2016). The current analysis is the first to evaluate the safety and clinical efficacy of enasidenib monotherapy in pts with mIDH2-positive MDS.
Methods: This analysis includes pts ages ≥18 years with MDS and mIDH2 who participated in a phase 1 study with a dose-finding period followed by an expansion phase in which all pts received daily oral enasidenib 100 mg QD in 28-day cycles. Pts had R/R MDS or were not candidates for standard therapies. Response was measured using peripheral blood (PB) and bone marrow (BM) samples from days 15, 29, 57, and every 56 days thereafter, and by objective investigator report. Overall response rate (ORR) reflects the best response achieved by pts, and includes complete remission (CR), partial remission (PR), marrow CR (mCR), and any hematologic improvement (HI) (IWG 2006 MDS criteria). Evaluable pts required a response assessment at Cycle 2 Day 1 or later, or discontinued before assessment. Overall survival (OS) was estimated using Kaplan-Meier methods. Next-generation sequencing identified pre-existing co-occurring genomic alterations using the FoundationOne® Heme test on purified mononuclear cells from BM or PB, to assess relationships between co-mutational status and clinical response.
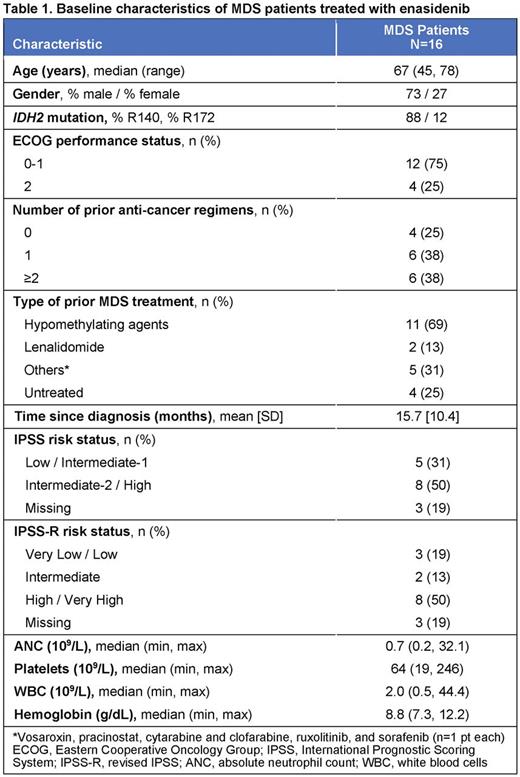
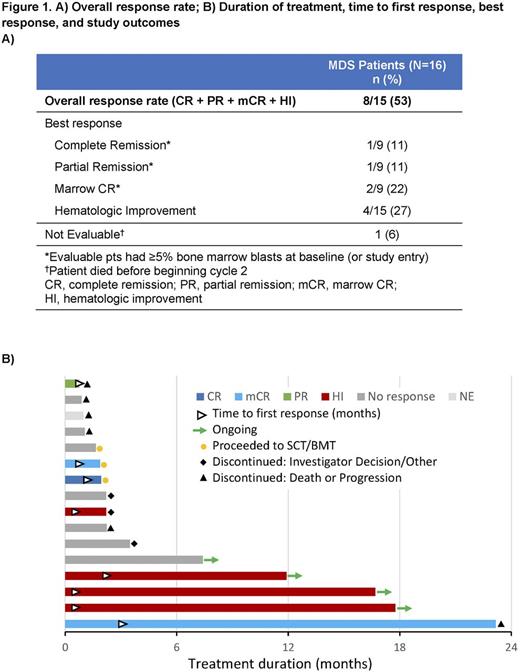
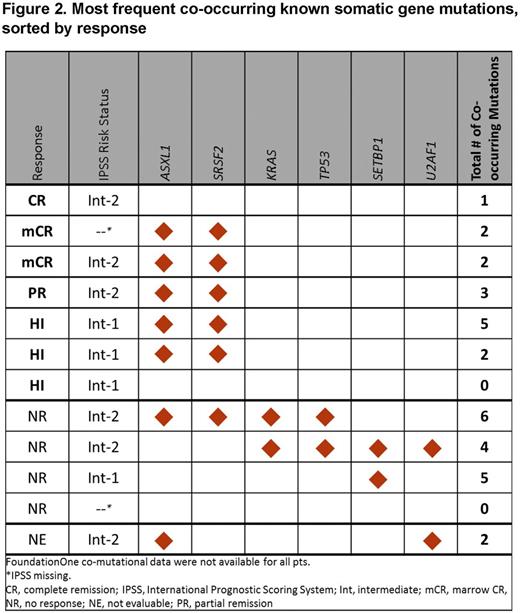
Results: Of 16 pts with MDS in this study, 12 pts had discontinued and 4 pts continued to receive enasidenib at interim database lock (15 April 2016). Reasons for discontinuation included disease progression (n=1), adverse event (AE; n=1), death (n=4), investigator decision (n=2), and other (n=1); 3 pts proceeded to transplant. Median age was 67 years (range 45-78) (Table 1). R140 mutations were more common than R172 mutations (88% vs 12%). At entry, 3 pts (19%) had relapsed following allogeneic stem cell transplant and 11 (69%) had failed prior treatment (Tx) with a hypomethylating agent (HMA). Six pts (38%) had received ≥2 prior anticancer Tx for MDS. MDS pts in the dose-finding phase received daily enasidenib doses of 60 mg (n=1), 150 mg (1), 200 mg (3), or 300 mg (1); 10 pts received enasidenib 100 mg QD. Median number of Tx cycles was 3 (range 1-25); 5 pts (31%) received ≥6 enasidenib cycles and 4 pts (25%) received ≥12 cycles. Grade 3-4 Tx-emergent AEs (TEAEs) were reported for 13 pts (81%); the most frequent were hyperbilirubinemia (n=5, unconjugated), pneumonia (n=4), thrombocytopenia (n=3) and hypokalemia (n=3). Seven pts (44%) had a grade 3-4 drug-related TEAE. One pt was not evaluable for response. ORR was 53% (8/15), including 1 pt who achieved CR (Figure 1). Of 10 evaluable pts who had received prior HMA Tx, 5 (50%) had a response with enasidenib, including the pt in CR. Of the 4 pts with no prior MDS Tx, 2 responded (1 PR, 1 mCR). Median time to CR, PR, or mCR (sustained ≥4 weeks) was 24 days (range 17-87) from beginning enasidenib Tx, and to HI (sustained ≥8 weeks) was 11 days (11-60). Two pts experienced disease progression. Median OS was not reached after a median follow-up of 4.7 months. FoundationOne® data were available for 12 pts; the most frequently observed known somatic co-occurring mutations were ASXL1 and SRSF2 (Figure 2). Although trends between response and co-occurring ASXL1 and/or SRSF2 mutations were observed, the small number of pts tested prevents definitive conclusions.
Discussion: Daily Tx with oral enasidenib monotherapy was well tolerated and induced responses in more than one-half of these MDS pts with mIDH2, 50% of whom had higher-risk disease, and two-thirds of whom had failed prior HMA Tx. Notably, one-half of evaluable MDS pts who had failed prior HMA Tx had a response, including a CR, with enasidenib monotherapy. Only 2 pts experienced disease progression during Tx. Mutational testing is rapidly becoming essential to diagnosis and prognostication in MDS, and assessment of IDH2 mutations can identify MDS pts who may benefit from targeted Tx with enasidenib.
Stein:Seattle Genetics: Research Funding; Agios Pharmaceuticals: Other: Advisory Board, Research Funding; Celgene: Other: Advisory Board, Research Funding; Novartis: Consultancy. Fathi:Merck: Other: Advisory Board participation; Agios Pharmaceuticals: Other: Advisory Board participation; Seattle Genetics: Consultancy, Other: Advisory Board participation, Research Funding; Celgene: Consultancy, Research Funding; Bexalata: Other: Advisory Board participation. DiNardo:Novartis: Other: advisory board, Research Funding; Agios: Other: advisory board, Research Funding; Celgene: Research Funding; Daiichi Sankyo: Other: advisory board, Research Funding; Abbvie: Research Funding. Pollyea:Celgene: Other: advisory board, Research Funding; Ariad: Other: advisory board; Glycomimetics: Other: DSMB member; Alexion: Other: advisory board; Pfizer: Other: advisory board, Research Funding. Roboz:Celgene: Consultancy; Astex: Consultancy; Agios: Consultancy; Pfizer: Consultancy; Juno: Consultancy; Genoptix: Consultancy; Amgen: Consultancy; MEI Pharma: Consultancy; Janssen: Consultancy; AstraZeneca: Consultancy; Onconova: Consultancy; Sunesis: Consultancy; Novartis: Consultancy; Roche/Genentech: Consultancy; MedImmune: Consultancy; Celator: Consultancy; Amphivena: Consultancy. Sekeres:Amgen Inc.: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Millenium/Takeda: Membership on an entity's Board of Directors or advisory committees. Stone:Pfizer: Consultancy; Sunesis Pharmaceuticals: Consultancy; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy; Amgen: Consultancy; Celator: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy; Novartis: Consultancy; Jansen: Consultancy; Juno Therapeutics: Consultancy; ONO: Consultancy; Seattle Genetics: Consultancy; Merck: Consultancy; Roche: Consultancy; Xenetic Biosciences: Consultancy. Attar:Agios Pharmaceuticals, Inc.: Employment, Equity Ownership. Tosolini:Celgene: Employment, Equity Ownership. Xu:Celgene: Employment, Equity Ownership. Amatangelo:Celgene: Employment, Equity Ownership. Gupta:Celgene: Employment, Equity Ownership. Knight:Celgene: Employment, Equity Ownership. De Botton:Agios, Celgene, Pfizer, Novartis, Pierre Fabre, Servier: Consultancy, Honoraria, Research Funding. Kantarjian:Amgen: Research Funding; ARIAD: Research Funding; Bristol-Myers Squibb: Research Funding; Pfizer Inc: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal