Abstract
Background: CMV disease is a major complication of allogeneic hematopoietic cell transplantation (HCT). Our widely used diagnostic modalities (H&E-based morphology and CMV IHC) are suboptimal in accuracy and frequently equivocal. Furthermore, current therapies for CMV disease are toxic and can cause serious complications such as prolonged neutropenia or progressive renal insufficiency. As a result, more accurate diagnosis that would help avoid potentially toxic treatments in patients who are truly negative for disease is highly desirable. The purpose of this study was to evaluate whether CMV PCR performed on formalin-fixed paraffin-embedded (FFPE) tissue offers additional diagnostic value to H&E/IHC in allogeneic HCT recipients.
Methods: Following the approval by our institutional review board, the electronic medical records of all adult patients who underwent an allogeneic HCT at Washington University School of Medicine (St. Louis, MO) between 2010 and 2015 and had a post-transplant upper/lower endoscopy were retrospectively reviewed. An additional inclusion criterion was the availability of CMV DNA PCR on the blood within 7 days of biopsy.FFPE specimens were reviewed by a pathologist who was blinded to clinical and molecular results. The specimens were reviewed for H&E-based morphology and IHC findings. Tissue PCR was performed by a laboratory technician who was blinded to clinical, H&E, and IHC findings.
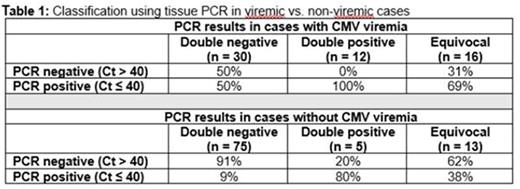
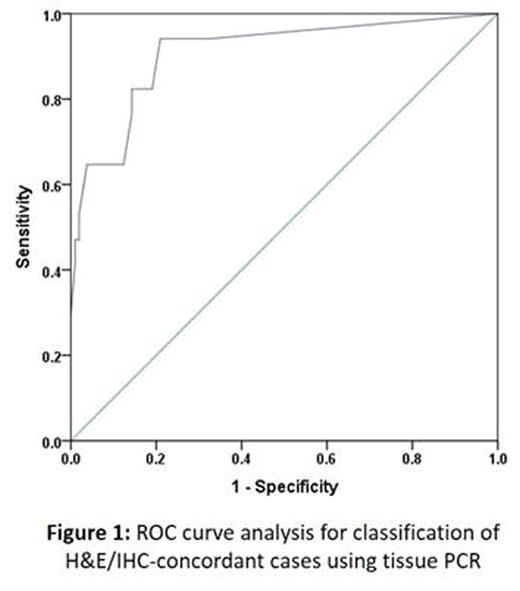
Results: A total of 151 samples were included. Concurrent CMV viremia was present in 38% of cases. According to H&E/IHC results, cases were classified as double-positive (n = 17), double-negative (n = 105), or equivocal (n = 29) (Table 1). In receiver operating characteristic curve analysis for classification of H&E/IHC-concordant cases using tissue PCR, the optimal cycle threshold (Ct) value was 40 (area under the curve = 0.91, P < 0.001, sensitivity 94%, specificity 79%, positive predictive value 42%, and negative predictive value 99%; Figure 1). Using this cutoff, 45% of equivocal cases were classified as negative, suggesting that anti-CMV treatment in almost half of H&E/IHC-equivocal cases is unnecessary and potentially detrimental. Among viremic, H&E/IHC-concordant cases, tissue PCR with a cutoff Ct of 40 had a sensitivity of 100%, specificity of 50%, PPV of 44%, and NPV of 100%. Among non-viremic, H&E/IHC-concordant cases, these numbers were 80%, 91%, 36%, and 99%, respectively. In this analysis on viremic cases, all double-positive and 50% of double-negative cases were classified positive, while 31% of equivocal cases were classified negative. In non-viremic patients, 91% of double-negatives, 20% of double-positives, and 62% of equivocal cases were classified negative.
Conclusions: Tissue PCR is a useful adjunct to H&E and IHC, particularly in H&E/IHC-equivocal cases, and can help avoid unnecessary, potentially toxic, anti-CMV treatment in cases without tissue-invasive disease. We propose the following algorithm: (i) If the cost and labor associated with tissue PCR on all patients are prohibitive, start with H&E and IHC. Perform tissue PCR in H&E/IHC-equivocal cases and consider this test also in non-viremic, H&E/IHC-positive cases. In all other cases forgo tissue PCR. (ii) If the cost and labor are not prohibitive, perform PCR on all cases. A negative PCR rules out CMV disease. In PCR-positive cases, use H&E/IHC results and treat only if H&E and IHC are both positive. One limitation of our study is related to using FFPE specimens rather than the real-life fresh tissue. Our results warrant testing in prospective studies.
DiPersio:Incyte Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal