Abstract
Background: Identifying factors that influence donor-derived immune response may ultimately enable its therapeutic redirection, lessening risk for complications following allogeneic hematopoietic cell transplantation (alloHCT) like acute graft-versus-host disease (aGvHD) and promoting protection against infection and malignant disease relapse. Viral reactivation seems poised to influence donor-derived immune response, potentially disrupting the balance between immune surveillance in eradicating malignancy and infection and immune tolerance in preventing aGvHD. Cytomegalovirus (CMV) is a clinically-significant virus with immunomodulatory capabilities. However, studies interrogating such effects are limited in the modern transplant era. The primary study aim was to estimate the cumulative incidence of initial CMV reactivation (RA) and aGvHD in alloHCT patients and to assess reciprocal influence between CMV RA and aGvHD. The secondary study aim was to define whether CMV RA predisposed alloHCT patients to infection or increased relapse risk.
Methods: Consecutive adult patients (n=324) with acute lymphoblastic leukemia, acute myelogenous leukemia or myelodysplasia whom received initial matched sibling or unrelated donor (MUD) bone marrow (n=33), peripheral blood stem cell (PBSC, n=253) or umbilical cord blood (n=38) grafts from January 2010 through December 2014 at The Ohio State University Comprehensive Cancer Center comprised the study cohort. Patient-, transplant-, and infection-related data were retrospectively analyzed (Table 1). Initial CMV RA was defined as plasma quantitative CMV PCR≥1000 viral copies/ml for which CMV-directed antiviral therapy was started. Microbiologically-documented infections were recorded for the first year after alloHCT and categorized as bacterial blood stream infection, invasive fungal infection, human herpes virus 6 viremia, and respiratory viral infection.
Cumulative incidences of CMV RA and aGvHD were estimated accounting for competing risks (death from any cause). Association between CMV RA and incidence of aGvHD was evaluated in a proportional sub-distribution hazards model, where CMV RA was treated as a time-dependent covariate with competing risk as death from any cause. Similarly, influence of aGvHD on incidence of CMV RA was evaluated where development of aGvHD was treated as the time-dependent covariate and CMVR RA as the end point of interest. Associations between CMV RA and subsequent infection or disease relapse were similarly analyzed. To evaluate impact of CMV and aGvHD on long-term outcomes, landmark analysis (LMA) at D100 and D365 were compared among four distinct patient groups: (1) No CMV RA, no aGvHD; (2) CMV RA, no aGvHD; (3) No CMV RA, aGvHD; and (4) CMV RA and aGvHD.
Results: Most transplant patients had AML in CR1, received MUD PBSC grafts following myeloablative conditioning, and were given methotrexate and calcineurin-based GvHD prophylaxis. Patients who developed aGvHD grades 2 (HR=1.93, 95% CI 1.15-3.23, p=0.013) or grades 3 and 4 (HR=3.36, 95% CI 1.76-6.45, p<0.001) had higher risk for developing subsequent CMV RA. In contrast, patients with initial CMV RA did not have increased risk for developing future aGvHD. Similarly, CMV RA did not have a significant impact on future infection, regardless of infection type, nor impact subsequent risk of malignant disease relapse.
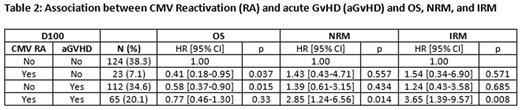
Overall survival (OS) at 1, 2, and 3 years post-transplant were 63%, 53%, and 45%, respectively. Cumulative incidence rates for non-relapse mortality (NRM) at 1, 2, and 3 years were 16%, 19%, and 23%, respectively; for infection-related mortality (IRM), cumulative incidence rates were 10%, 13%, and 16%, respectively. OS, NRM and IRM were stratified by initial CMV RA and aGvHD status at D100 and D365 (Table 2). Among patients who were alive, patients whom had initial CMV RA and aGvHD by D100 were at higher risk for NRM (HR=2.85, 95% CI 1.24-6.56, p=0.014) and IRM (HR=3.65, 95% CI 1.39-9.57, p=0.008) than patients who experienced neither CMV RA nor aGvHD. D365 LMA did not reveal any statistically significant differences in OS, NRM, and IRM between groups.
Conclusion: aGvHD associated with increased risk for CMV RA, but initial CMV RA did not associate with subsequent aGvHD risk. Furthermore, initial CMV RA did not associate with increased risk for disease relapse or infection, but did increase NRM and IRM, particularly in combination with aGvHD.
Auletta:Shire Pharmaceuticals: Consultancy. Lozanski:Genentech: Research Funding; Stemline Therapeutics Inc.: Research Funding; Boehringer Ingelheim: Research Funding; Beckman Coulter: Research Funding. Hofmeister:Janssen: Pharmaceutical Companies of Johnson & Johnson: Research Funding; Karyopharm Therapeutics: Research Funding; Signal Genetics, Inc.: Membership on an entity's Board of Directors or advisory committees; Incyte, Corp: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Arno Therapeutics, Inc.: Research Funding; Takeda Pharmaceutical Company: Research Funding; Teva: Membership on an entity's Board of Directors or advisory committees. Andritsos:Hairy Cell Leukemia Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal