Abstract
Aim
Relapse remains the leading cause of death for patients with acute myeloid leukemia (AML). Some patients at high risk of relapse are ineligible for allogeneic stem cell transplantation due to age, comorbidities or the absence of a suitable donor. Infusion of fresh haploidentical cells from family donors (microtransplantation) has resulted in improvement in disease-free survival without the development of graft-versus-host disease (GVHD). However, obtaining family donor haploidentical G-CSF primed cells at short notice places a strain on transplant centres because of the requirement for rapid donor evaluation, testing and apheresis. In a Phase I study, we investigated the use of cryopreserved partially HLA-matched cells from unrelated donors as an immediately available alternative to family donors in AML patients ineligible for transplant.
Method
A bank of G-CSF primed and steady state apheresis products from 31 normal stem cell transplant donors that were no longer required for their originally intended recipients was established at two transplant centres. Patients in first complete remission (CR) of AML following induction therapy who were not candidates for allogeneic transplant were infused with partially HLA-matched (2/8 HLA-A, -B, -C or DR antigens) unrelated donor apheresis products at a dose of 0.5 x108 CD3 cells/kg following 3 days of cytarabine 2g/m2 BD. No GVHD prophylaxis was given. Up to three cycles were given at 2-month intervals. The presence of microchimerism was assessed via droplet digital PCR using primers/probes targeting 29 bi-allelic insertion/deletion loci.
Results
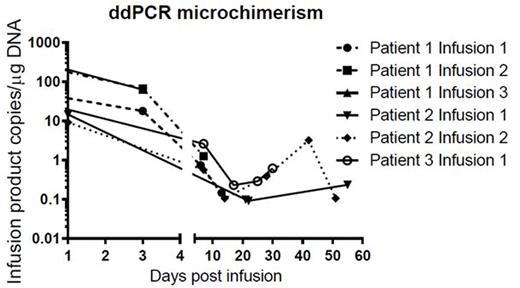
A total of eight cell infusions have been administered to three patients (Table 1). Non-neutropenic fever occurred within 24 hours of 5 of 8 infusions. Two febrile reactions were associated with transient rashes that were not consistent with GVHD on biopsy. These resolved spontaneously. There were no cases of GVHD following infusion. Median time to neutrophil recovery was 21.5 days (range 13-34) and platelets 17 days (9-39) post commencement of chemotherapy. One patient relapsed 256 days after his 3rd infusion and subsequently died, the other two remain in CR on trial. Of six infusions analysed, microchimerism has been detected in all cases up to 57 days post infusion (Figure 1).
Conclusion
Infusion of partially HLA-matched cells from unrelated donors results in minimal toxicity and engraftment of donor cells lasting up to 2 months post-infusion. Early non-neutropenic fevers and rashes are common. The effect of donor cells on disease recurrence will be determined through recruitment of additional patients and longer follow up.
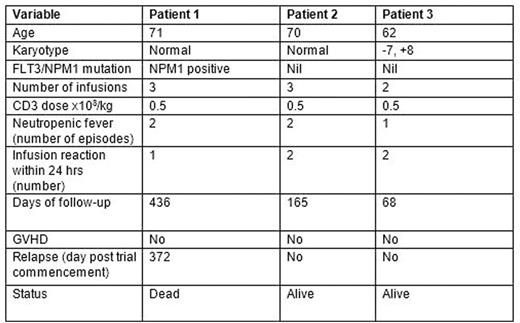
Patient details
Donor DNA detected following partially HLA matched unrelated donor cell infusions
Donor DNA detected following partially HLA matched unrelated donor cell infusions
Gottlieb:Abbvie: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Indee: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal