Abstract
Foxp3+ regulatory T cells (Tregs) are important regulators of graft-versus-host disease (GvHD) pathogenesis and a potential cellular therapy to prevent and treat GvHD. Thymus-derived (t)Tregs, while less frequent, are effective in preventing GvHD by ex vivo expansion, however Ag-non-specificity, stability, and cost-effectiveness are still critical issues to overcome. Peripherally-induced (p)Tregs are readily generated in large numbers in vitro;however, they are not suitable for therapeutic usage due to their rapid loss of Foxp3 expression and suppressive function when infused in the context of GvHD. The development of new approaches to increase in vivo stability/suppressive function of pTregs for clinical use is thus warranted.
We recently demonstrated that IL-27 stimulation enhances suppressive function of Tregs (both tTregs and pTregs) in vitro and in vivo (Do, et al. Mucosal Immunol 2016). We hypothesized that IL-27 pre-stimulation would enhance pTreg function to suppress acute GvHD. Naïve CD4 T cells (from Foxp3GFP mice) were stimulated with anti-CD3/CD28 mAbs in the presence of TGFβ1 and IL-2 for 3 days, and >90% of the cells expressed GFP. Cells were then re-stimulated with the Abs for 3 more days with or without IL-27. IL-27 pre-stimulation enhanced Treg suppressive function in vitro, and was superior to that of rapamycin (Figure 1A). Lethally irradiated BALB/c mice received B6 BM cells with or without purified B6 CD3+ T cells. IL-27 pre-stimulated and control GFP+ pTregs were FACS sorted and transferred at the time of reconstitution. GvHD was evaluated by clinical features and survival. BM recipients that received T cells alone succumbed to death within 2 weeks of reconstitution due to lethal acute GvHD. While only ~40% of the control pTreg recipients were protected from lethal GvHD, all IL-27 pre-stimulated pTreg recipients were protected and survived (Figure 1B). CD4 and CD8 T cell expansion and ex vivo IFNγ production was substantially diminished with IL-27 pre-stimulated pTregs (Figure 1C). Next, lymphoma A20 cells transduced with the luciferase were transferred together with BM cells. Tumor growth was monitored. BM alone recipients had progression of tumor growth by day 14. Recipients of T cells alone died of lethal acute GVHD prior to day 14. Recipients of control and IL-27 stimulated pTregs had complete elimination of tumor cells. Of note, only 3 out of 5 recipients of control pTregs survived, with 2 succumbing to lethal GVHD prior to day 14 (Figure 1D). Although the total numbers of IL-27 pre-stimulated pTregs were higher than control pTregs, this was not statistically significant. GFP (Foxp3) expression of transferred pTregs, however, was markedly increased in IL-27 stimulated cells.
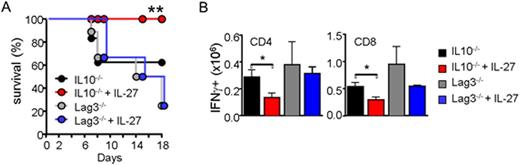
We previously reported that IL-27 signaling in Tregs upregulates IL-10 and Lag3 (Do, et al. Mucosal Immunol 2016). We thus generated Il10-/- and Lag3-/- pTregs. Foxp3 expression of these pTregs was comparable to that of wild type pTregs, and IL-27 stimulation did not affect Foxp3 expression. IL-27-mediated enhanced in vitro suppression was lost in Lag3-/- but not in Il10-/- Tregs. Following IL-27 pre-stimulation, Il10-/- and Lag3-/- pTregs were transferred into recipients induced for acute GvHD. Foxp3 expression was similar at the time of transfer. IL-27 pre-stimulated Il10-/- pTregs protected recipients from acute GvHD lethality, however, Lag3-/- pTregs failed to protect from GVHD, even after IL-27 pre-stimulation (Figure 2A). IFNγ production of donor T cells was significantly reduced by IL-27 pre-stimulated Il10-/- but not by Lag3-/- pTregs (Figure 2B).
In conclusion, we report that IL-27 pre-stimulation greatly enhances pTreg function, preventing acute GvHD lethality, while preserving a graft-versus-leukemia (GvL) effect. Lag3 on pTregs appears to be a key molecule mediating pTreg suppressive function enhanced by IL-27.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal