Abstract
Introduction: Renal insufficiency is common in multiple myeloma (MM). Carfilzomib (CFZ), an epoxyketone proteasome inhibitor, is approved in the United States for the treatment of relapsed or refractory MM in patients (pts) who have received 1-3 prior lines of therapy. A previous study (PX-171-005) of pts with MM found no apparent differences in CFZ (15 or 20 mg/m2) clearance, area under the concentration time curve (AUC), and maximum plasma concentration (Cmax) between pts with normal vs those with mild, moderate, or severe renal impairment. The primary objective of this study was to assess the influence of end stage renal disease (ESRD) on the pharmacokinetics (PK) of 56 mg/m2 CFZ in pts with relapsed MM.
Methods: Pts with relapsed MM (≥1 prior lines of therapy ) with normal renal function (fcn; creatinine clearance ≥75 mL/minute [min]) or ESRD requiring hemodialysis received CFZ infusion (over 30 min) on 2 consecutive days each week (wk) for 3 wks (days 1, 2, 8, 9, 15, and 16) every 28-day cycle; 20 mg/m2 on day 1 to 2 of cycle 1; escalated to 27 mg/m2 on day 8 of cycle 1; if tolerated, 56 mg/m2 started on day 1 of cycle 2. Pts also received dexamethasone 8 mg/day during cycles 1 to 2 on the same days as CFZ, with dexamethasone optional for cycles ≥3. PK parameters were evaluated using a non-compartmental approach. The CFZ PK of pts with ESRD and normal renal fcn were compared using summary statistics and analysis of variance of ln-transformed PK parameters. The primary endpoints were AUC (from time 0 to last concentration measured [AUC0-last] and time 0 extrapolated to infinity [AUC0-inf]) of CFZ 56 mg/m2 on day 1 of cycle 2. Additional endpoints included Cmax, time to maximum concentration (Tmax), clearance (CL), terminal half-life (T1/2), volume of distribution at steady state (Vss), mean residence time (MRT), and safety and tolerability of CFZ and overall response rate (ORR; defined as partial response [PR] or better) in pts who received ≥1 CFZ dose.
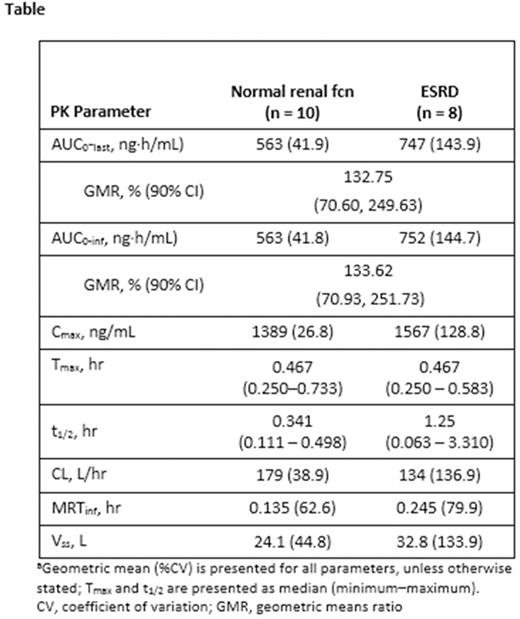
Results: A total of 26 pts were treated; 15 normal renal fcn and 11 ESRD on hemodialysis. Demographic and baseline characteristics were generally balanced between the 2 cohorts; 65.4% had an ECOG performance score of 1, were a median of 4.2 years from initial diagnosis, and had a median of 3 prior therapies. Ten pts with normal renal fcn and 8 with ESRD were evaluated for the primary endpoint at the 56 mg/m2 dose. ESRD pts demonstrated a trend toward a higher AUC, Cmax and half-life (t1/2), and a slower CL compared with normal renal fcn pts (Table). However, the inter-subject variability in CFZ exposure (AUC and Cmax) was high, with percent coefficient of variations (%CVs) >100% in ESRD pts. The mean CFZ systemic exposures (AUC0-last and AUC0-inf) were approximately 30% higher in ESRD vs normal renal fcn pts (Table). Exposure to some CFZ metabolites was elevated in pts with ESRD; however, these metabolites have no biologic activity. A total of 24 pts were evaluable for response (14 normal renal fcn, 10 ESRD). ORR for the entire study population was 50% (95% CI: 29.1, 70.9); 60% (95% CI: 26.2, 87.8) in the ESRD group and 43% (95% CI: 17.7, 71.1) in the normal renal fcn group. At data cutoff (10/12/15), median CFZ treatment duration was 14.1 wks (range, 2.3-87.1), with a median of 4.0 cycles (range, 1-4). Median exposure was 14.1 (4.0 cycles) and 12.1 (3.0 cycles) wks for pts with normal renal fcn vs ESRD, respectively. All patients had treatment-emergent adverse events (TEAE); grade ≥3 TEAE occurred in 12/15 (80%) normal renal fcn pts and 9/11 (82%) ESRD pts. Most common grade ≥3 AEs were thrombocytopenia, anemia, and pneumonia. Six (23%) pts with normal renal fcn and 0 with ESRD pts discontinued CFZ due to TEAEs. There were a total of 5 deaths during the study (1 cardiac arrest [normal renal fcn], 4 progressive disease [2 per cohort]); none were considered related to CFZ.
Conclusions: Following a 30-min infusion of 56 mg/m2 CFZ, there was a trend toward a higher AUC, Cmax, and t1/2, and a slower clearance in pts with ESRD vs pts with normal renal fcn. However, the trend differences were smaller than between-subject PK variability. The clinical benefit of therapy with CFZ was not adversely affected by ESRD. The observed AE profile in pts with ESRD was similar and generally consistent with the known safety profile of CFZ in the treatment of pts with relapsed MM. Based upon PK data and safety results, no dose adjustment of CFZ appears to be warranted in pts with MM and ESRD or pts with varying degrees of renal impairment.
Quach:Amgen: Membership on an entity's Board of Directors or advisory committees; Janssen Cilag: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. White:Amgen: Honoraria. Ho:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. White:Amgen: Employment. Inamdar:Amgen: Employment. Morris:Amgen: Employment. Ou:Amgen: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal