Abstract
Introduction: Daratumumab is a human CD38 IgGκ monoclonal antibody that demonstrated significant activity and a manageable safety profile in combination with bortezomib and dexamethasone. In a randomized phase 3 study, daratumumab in combination with bortezomib and dexamethasone (DVd) significantly prolonged progression-free survival (PFS) versus bortezomib and dexamethasone alone (Vd) in a pre-specified interim analysis of patients (pts) with relapsed or refractory multiple myeloma (RRMM; Palumbo A. N Engl J Med 2016; in press). Herein, we examine subgroups from this study to compare the efficacy of DVd vs Vd in bortezomib-naive and bortezomib-experienced pt populations. In addition, the efficacy of DVd vs Vd in pts who were refractory to lenalidomide at last prior line of therapy was also evaluated.
Methods: Pts who received ≥1 prior line of therapy were randomized (1:1) to 8 cycles (q3w) of Vd (bortezomib: 1.3 mg/m2 SC on Days 1, 4, 8, 11; dexamethasone: 20 mg PO on Days 1, 2, 4, 5, 8, 9, 11, 12) with or without daratumumab (16 mg/kg IV qw in Cycles 1-3, Day 1 of Cycles 4-8, then q4w until progression). Pts who were refractory to bortezomib were not eligible. The primary endpoint was PFS. Bone marrow aspirate samples that had been prepared with Ficoll were evaluated for minimal residual disease (MRD) using three different thresholds (10-4, 10-5, and 10-6) based on the ClonoSEQ assay (Adaptive Biotechnologies, Seattle, WA, USA).
Results: Median follow-up was 7.4 months. Among bortezomib-naive pts (DVd, n=89; Vd, n=83), PFS was significantly improved with DVd vs Vd (median: not reached [NR] vs 7.5 months; HR, 0.25; 95% CI, 0.13-0.47; P<0.0001); estimated 12-month PFS rates were 72% vs 28%, respectively. ORR was 88% with DVd vs 70% with Vd (P=0.0040), with ≥very good partial response (VGPR) rates of 72% vs 42% (P<0.0001), and ≥complete response (CR) rates of 30% vs 20% (P=0.1199), respectively.
For pts who previously received a bortezomib-containing regimen (DVd, n=162; Vd, n=164), PFS was also significantly longer with DVd vs Vd (median: 12.3 vs 6.7 months; HR, 0.46; 95% CI, 0.32-0.66; P<0.0001). Estimated 12-month PFS rates were 55% vs 27%, respectively. ORR was significantly higher with DVd vs Vd (80% vs 60%; P=0.0001), along with significantly higher rates of ≥VGPR (52% vs 22%; P<0.0001) and ≥CR (13% vs 3%; P=0.0019).
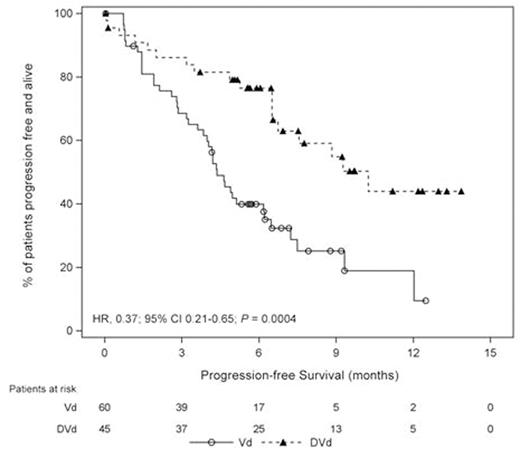
Among pts who were refractory to lenalidomide at the last prior line of therapy (DVd, n=45; Vd, n=60), PFS was significantly longer in DVd vs Vd (median: 10.3 vs 4.4 mo; HR, 0.37; 95% CI, 0.21-0.65; P=0.0004; Figure). Within this subgroup, ORR was significantly higher for DVd vs Vd (81% vs 50%; P=0.0021), and the same trends were observed for rates of ≥VGPR (54% vs 12%; P<0.0001) and ≥CR (20% vs 5%; P=0.0261).
Updated efficacy and safety data, including MRD analyses across different sensitivity thresholds (10-4, 10-5, and 10-6), will be presented at the meeting.
Conclusions: These analyses confirm that addition of daratumumab to Vd significantly improves outcomes for RRMM pts regardless of prior treatment with bortezomib. Importantly, this treatment benefit of DVd vs Vd was maintained in pts who were refractory to lenalidomide at the last prior line of therapy. These data lend further support to adding daratumumab to a standard-of-care regimen in RRMM.
Progression-free Survival in Patients Refractory to Lenalidomide at the Last Prior Line of Therapy
Progression-free Survival in Patients Refractory to Lenalidomide at the Last Prior Line of Therapy
Lentzsch:BMS: Consultancy; Foundation One: Consultancy; Celgene: Consultancy, Honoraria. Quach:Celgene: Membership on an entity's Board of Directors or advisory committees; Janssen Cilag: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Ovilla:Janssen: Consultancy. Qi:Janssen: Employment. Deraedt:Janssen: Employment, Equity Ownership. Schecter:Janssen: Employment, Equity Ownership. Amin:Janssen: Employment. Qin:Janssen: Employment. Casneuf:Johnson & Johnson: Equity Ownership; Janssen R&D, Beerse, Belgium: Employment. Chiu:Janssen: Employment. Sasser:Johnson & Johnson: Equity Ownership; Janssen Pharmaceuticals R&D: Employment. Sonneveld:Karyopharm: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal