Abstract
Background: XPO1 (Exportin-1/CRM1) is the non-redundant nuclear exporter of over 200 cargos including the major tumor suppressor proteins. Deregulated nuclear export by changes in XPO1 expression is a common characteristic for a broad range of cancers and may aid in the evasion of anti-neoplastic mechanisms. As a result, inhibition of XPO1 has emerged as a promising area of cancer treatment. The Selective Inhibitor of Nuclear Export (SINE) compounds, selinexor, as well as a second generation, KPT-8602, bind to the XPO1 cargo binding pocket and disrupt XPO1-mediated nuclear export, resulting in cancer specific cell death. Although selinexor has been evaluated in >1,500 patients and has manageable tolerability, KPT-8602 may have improved tolerability and efficacy based on decreased brain penetration in animal models allowing more frequent dosing. Currently, the safety, tolerability and efficacy of KPT-8602 is being evaluated in a phase 1 trial of patients with relapsed/refractory multiple myeloma (MM; NCT02649790). Since selinexor synergizes with a broad array of anti-MM agents in patients, KPT-8602 is an excellent candidate for combination therapies. In this study, we investigated the use and mechanism of combining KPT-8602 with the pan-histone deacetylase (HDAC) inhibitor, panobinostat, in MM cell lines and in a xenograft mouse model of MM.
Methods: MM.1S cells were treated with single agent KPT-8602, panobinostat or a combination of both. The effects of KPT-8602 and/or panobinostat on cell viability were examined using standard viability assays after 72 hours of treatment. Total RNA or protein levels were examined after 24 hours using quantitative PCR or immunoblots, respectively. Immune compromised mice were injected subcutaneously with MM.1S cells. Tumors were allowed to grow to ~150 mm3before treatment. The mice were treated with vehicle, sub-therapeutic doses of KPT-8602 (5 mg/kg PO QDx5) or panobinostat (5 mg/kg IP QDx5) alone or in combination. Tumor growth and animal weights was monitored to determine tumor growth inhibition (TGI), tumor regression, and tolerability to treatment. The tumors were then harvested for immunohistochemical (IHC) analysis.
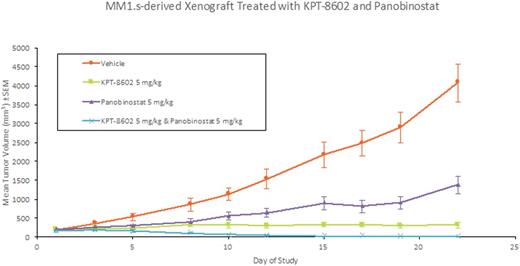
Results: The combination of KPT-8602 and panobinostat was highly effective against MM.1S cell viability. A synergistic anti-cancer effect was observed against MM.1S cells grown in culture and in mice. In cells, the MTT IC50of KPT-8602 was shifted from 50 to 23 nM by the addition of sub cytotoxic concentrations of panobinostat. In mice, single agent treatment with KPT-8602 led to 96.5% tumor growth inhibition whereas panobinostat resulted in 69.4% tumor growth inhibition within 22 days. Remarkably, in the combination of KPT-8602 and panobinostat, 3 out of 8 tumors totally disappeared and the overall tumor regression was 95%, (Figure 1). Both drugs, as single agents and in combination were well tolerated and no significant changes in weight were observed. Gene and protein expression studies revealed that although both compounds target independent proteins (e.g. HDACs or XPO1), the combination significantly enhances markers of cell death (cleavage of PARP-1, caspase-3, etc.). Curiously, KPT-8602 enhances the inhibitory effect panobinostat has on deacetylation as evidenced by histone acetylation. Moreover, DNA damage, as indicated by ϒ-H2AX, significantly increases in the presence of both compounds.
Conclusion: KPT-8602 and panobinostat are dissimilar drugs with unique mechanisms of action, and individually affect a broad range of cellular processes. Here we show that the combination of these drugs can dramatically increase the already potent anti-cancer properties of these compounds in MM cell lines. In addition, KPT-8602 enhances the inhibitory effect exerted by panobinostat on histone deacetylation, which coincides with an increase induction of DNA damage. It should be noted that both panobinostat and SINE compounds have been shown to downregulate checkpoint and DNA damage response (DDR) proteins (e.g. RAD51 and Chk1). We hypothesize that the combination of KPT-8602 and panobinostat promotes significant chromatin remodeling in the presence of a compromised DDR pathway, which destabilizes genomic integrity in MM cells and leads to a synergistic effect on cell viability. Together, these data provide rational support for the study of KPT-8602 and panobinostat in clinical trials.
Argueta:Karyopharm Therapeutics: Employment, Equity Ownership. Chang:Karyopharm Therapeutics: Employment, Equity Ownership. Kashyap:Karyopharm Therapeutics: Employment, Equity Ownership. Elloul:Rubius Therapeutics: Employment. Friedlander:Karyopharm Therapeutics: Employment. Lee:Karyopharm Therapeutics: Employment, Equity Ownership. Kauffman:Karyopharm Therapeutics Inc: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Shacham:Karyopharm Therapeutics: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Senapedis:Karyopharm Therapeutics: Employment, Equity Ownership. Baloglu:Karyopharm Therapeutics: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal