Abstract
Background: The detection of minimal residual disease (MRD) in CLL using a 0.01% cut-off is an independent predictor of progression-free (PFS) and overall survival (OS) after chemo-immunotherapy. There are several new treatments available and using MRD as a trial endpoint could greatly speed up the identification of the best treatment approaches. MRD assessments can be made in the peripheral blood (PB) or bone marrow (BM) but there is substantial variation in the extent to which different treatments deplete disease in the PB, BM, and nodal compartments.
Aims: To compare MRD levels in the PB & BM during/after different treatment approaches to determine differential compartment effects and evaluate the impact on predicting PFS/OS.
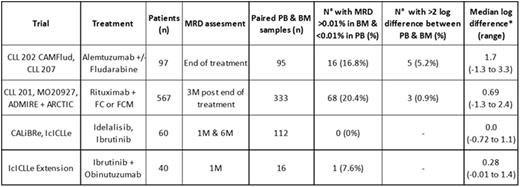
Methods: The level of residual disease was determined using multi-parameter flow cytometry according to ERIC consensus protocols with a limit of quantification of 10-4 / 0.01% or better; "+" and "-" denotes ≥0.01% and <0.01% MRD respectively. MRD assessment in the CAMFlud and CLL 207 (alemtuzumab-based) trials was performed at end of treatment because cessation was determined by MRD status. In the ADMIRE/ARCTIC (FCR-based) trials, the MRD assessment was 3 months after end of treatment. In the CALiBRe (idelalisib) & IcICLLe (ibrutinib±obinutuzumab) trial, MRD assessments were made during treatment.
Results: The MRD level in PB & BM samples taken at the same time, either during or within 6 months of treatment, was available in 556 cases of which 85 (15%) showed discordant results at the 0.01% threshold. The frequency of discrepant PB & BM MRD levels varied across treatment and patient groups (table 1). In 322 evaluable cases from the ADMIRE/ARCTIC trials at 3 months after the end FCR-based therapy, 66/322 were BM+PB-, representing 54% of the total MRD+ cases. Discrepant results with >1 log difference between PB & BM MRD levels were most common in refractory patients treated with alemtuzumab. There were no discrepant results in patients undergoing idelalisib or ibrutinib monotherapy although all patients have ≥0.01% MRD to date. A small compartment effect was apparent in patients treated with a combination of ibrutinib and obinutuzumab but the relative difference in PB vs. BM disease level appears to be approximately half that of rituximab in combination with chemotherapy.
To determine whether the discrepant results affect the ability of MRD status to predict outcome, we compared the PFS according to BM & PB MRD status. In the CLL202 (fludarabine + alemtuzumab) trial the PFS for BM+PB- cases was similar to BM+PB+ cases (median PFS was 1.6 and 6.5 months respectively) and both were substantially shorter than for BM-PB- cases (median PFS 44.7 months, Log-rank P<0.001). This contrasts with FCR-based therapy in the ADMIRE/ARCTIC trials where the PFS for BM+PB- cases was most similar to BM-PB- cases and both were substantially longer than for BM+PB+ cases (median PFS >66 months vs. 54 months vs. 33 months for BM-PB- vs. BM+PB- vs. BM+PB+ respectively, Log-rank P<0.001). BM MRD was the most sensitive and informative site for predicting outcome, although MRD status in both PB & BM were strong predictors of PFS and OS. All patients with >1% MRD in the PB also had >1% disease in the BM.
Summary/Conclusion: The compartment effect varies greatly according to patient group and treatment. Rituximab typically results in 0.7log lower disease in the PB compared to BM. Patients on BCR-pathway inhibitors have similar levels of disease in PB & BM even in combination with obinutuzumab. This suggests either that BCR-pathway inhibitors counteract the compartment effects of anti-CD20 antibody treatment, or that obinutuzumab is more effective in depleting bone marrow disease than rituximab. Peripheral blood MRD status may be appropriate for predicting outcome in some settings but underestimates MRD level in a substantial proportion of patients. Bone marrow remains the most sensitive and reliable source for MRD detection but is not required if there is >1% MRD in the blood. Evaluating the compartment effect of specific treatment combinations will help to identify approaches to achieve maximal MRD response using peripheral blood monitoring.
Compartment effect - difference in MRD detection in PB vs. BM samples across different trials.
* median log difference only calculated in cases with >0.01% MRD in both PB & BM so may underestimate compartment effect.
Compartment effect - difference in MRD detection in PB vs. BM samples across different trials.
* median log difference only calculated in cases with >0.01% MRD in both PB & BM so may underestimate compartment effect.
Rawstron:AbbVie: Honoraria; Roche: Honoraria; Celegene: Honoraria; Janssen: Research Funding; BD Biosciences: Other: Remuneration; Gilead: Consultancy, Honoraria, Research Funding; Genzyme: Honoraria; GlaxoSmithKline: Honoraria. Munir:Alexion pharmaceuticals: Honoraria. Brock:AstraZeneca: Equity Ownership; GlaxoSmithKline: Equity Ownership. Hillmen:Pharmacyclics: Research Funding; Janssen: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Gilead: Honoraria, Research Funding; Abbvie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal