Abstract
Introduction: Therapy-related myeloid neoplasms (T-MN; inclusive of T-MDS and T-AML) are aggressive neoplasms occurring after exposure to chemo (CT) and/or radiotherapy (RT) and are characterized by poor prognosis. Unlike primaryMDS and AML, the mutational architecture of T-MN has not been well elucidated. Most published studies in T-MN are small in size with a restricted panel of genes tested. Here we compare the cytogenetic and mutation profile of T-MN and primary MDS patients from the South Australian Myelodysplastic Syndrome (SA-MDS) registry.
Methods: Demographic, clinical and laboratory data including cytogenetic profile of 147 T-MN and 744 primary MDS patients were analysed. Targeted Massively Parallel Sequencing of a custom panel of 27 myeloid genes (all coding regions) was performed on bone marrow samples from 60 T-MN and 142primary MDS patient samples.
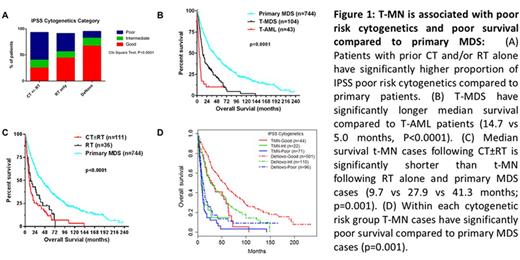
Results: Median age of T-MN and primary MDS was 71 (20-91) and 73 years (19-98), respectively. In T-MN patients the most frequent primary neoplasms were lymphoproliferative neoplasms (n=56, 38%) and prostate cancer (n=22, 15%). Sixty-three (43%) patients had received CT only, 34 (23%) patients RT only, and 48 (33%) patients had received both CT and RT. The T-MN group consisted of 104 T-MDS (71%) and 43 T-AML (29%). Poor risk cytogenetics were more frequent in T-MN cases following CT than after RT alone, and more frequent than in primary MDS cases (52% vs 25% vs 13%; P<0.0001 Fig 1A). Overall survival (OS) of T-MN cases was shorter than in primary MDS (P=0.001; Fig 1B). Within the T-MN group, T-AML cases had shorter OS than T-MDS cases (14.7 vs 5.0 months, P<0.0001; Fig 1C). Within each International Prognostic Scoring System (IPSS) cytogenetic risk group the T-MN patients had shorter OS than primary MDS patients (Fig 1D).
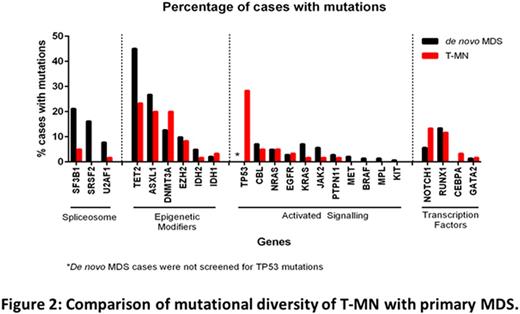
Mutation profiling detected at least one mutation in 117/142(82%) of primary MDS cases (total 324 mutations), similar to previous reports. Contrary to the published literature (42-58%; Shih et al, Haematologica 2013 & Ok CY et al, Leuk Res 2015), a high proportion of T-MN (54/60; 90%) patients showed at least 1 mutation. Notably, 14/15 (93%) of normal karyotype T-MN cases harbored at least one mutation. Although frequency of mutation was similar between T-MN and primary MDS cases, mutation burden and pattern were different (Fig 2). Multiple mutations were detected in 75% of primary MDS compared to 48% of T-MN cases. Mutations in spliceosome complex genes were detected in 44% of primary MDS cases, with SF3B1 being most common, while only 7% of T-MN cases (4/60) harboured these mutations. Mutations in TET2, ASXL1, NOTCH1, and RUNX1 were less frequent in T-MN compared to primary MDS (Fig 2). Mutations in TP53 were detected in 17/60 T-MN cases (28%), were associated with del7q/complex/monosomal karyotypes (15/17; 88%) and with prior CT±RT exposure.
We then compared mutation frequency between T-AML and T-MDS cases. The proportion of patients with at least one mutation (89% vs 93%) and the mean number of mutations per case (1.84 vs 1.57) were similar. The genes in which mutations occurred were different: DNMT3A was more frequent in T-AML (36% vs 15%), while NOTCH1 (17%), SF3B1 (7%), and EZH2 (11%) mutations were seen only in T-MDS.
Multiple somatic mutations in the same gene were detected in 30% (42/142) and 18% (11/60) of primary MDS and T-MN cases, respectively; most often in TET2, ASXL1, KRAS, NRAS, and TP53. In 41% (13/32) primary MDS and 28% (2/7) of T-MN cases harboring multiple somatic TET2 mutations, the variant allele frequency was similar (40-50%) suggesting biallelic clonal origin.
Conclusions: Contrary to published literature, mutation frequency in our study is higher in T-MN and is similar to primary MDS. Though frequency of mutation is similar in T-MN and primary MDS, mutation pattern was different. Unlike primary MDS, mutations in the spliceosome complex were rare in T-MN while TP53 mutations were detected in 28% of T-MN cases. Multiple somatic mutations in the same gene were detected in 30% primary MDS and 18% T-MN cases. In summary, this study highlights differences in cytogenetic and mutation profiling between primary MDS and T-MN, and provides insight into molecular pathogenesis of T-MN.
Ross:BMS: Honoraria; Novartis Pharmaceuticals: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal