Abstract
Background: Ruxolitinib is a JAK1/2 inhibitor that gained FDA approval for treatment of intermediate and high-risk myelofibrosis (MF) in 2011. Prior to ruxolitinib, a variety of treatments were used for myelofibrosis, with variable efficacy. Even in the ruxolitinib era, treatment decisions are primarily focused on symptom management, as no treatment, other than allogeneic hematopoietic stem-cell transplant (allo-HSCT) has shown to have a strong disease-modifying effect. Ruxolitinib significantly improves splenomegaly and constitutional symptoms associated with MF. Here, we aim to compare MF treatments before and after ruxolitinib approval to assess treatment patterns and impact on clinical outcomes. We hypothesized that an increased use of ruxolitinib after its FDA-approval would affect those with constitutional symptoms and/or splenomegaly and correlate with decreased use of other treatments aimed at these symptoms.
Methods: This was a single institution, retrospective study of all patients with a diagnosis of MF who were seen at our center between 2/2001 and 6/2016. The World Health Organization 2016 definition of primary myelofibrosis (PMF) was used for confirmation of diagnosis. Initial treatment was defined as first treatment after diagnosis of MF. Specific phenotypes were assigned retrospectively based on chart review reflecting the patient's initial complaints which led to diagnosis.
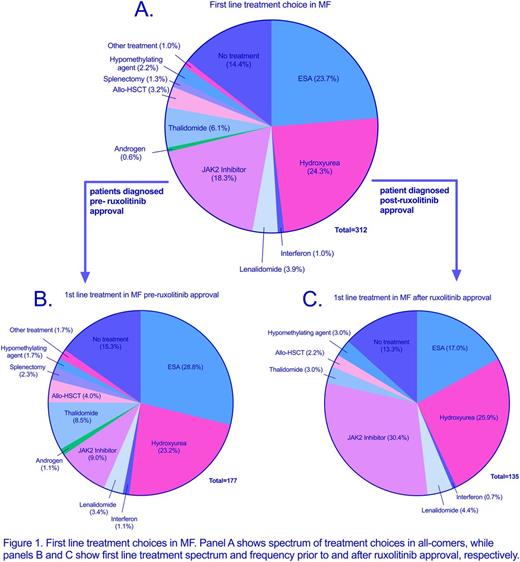
Results: We identified 312 eligible patients. This group was divided into two cohorts: those diagnosed prior to the ruxolitinib era (cohort PRE, n = 177) and those diagnosed in the ruxolitinib era (cohort POST, n = 135). Demographics (gender, race, age at diagnosis) and presenting features were comparable between the cohorts. In the PRE cohort, JAK2 inhibitor use occurred in the setting of a clinical trial as well as in patients who were diagnosed prior to ruxolitinib approval, but first received treatment after its approval. In this cohort, 25% of patients received a JAK2 inhibitor, with 36% of these patients receiving it as frontline therapy (see figure 1). This compared to 44% of POST patients who received a JAK-2 inhibitor, with 69% receiving it in the frontline setting. POST patients were, more likely to receive ruxolitinib overall (OR 2.28, p = 0.001) and as first-line therapy (OR 4.49, p < 0.0001). POST patients were less likely to receive an erythropoiesis-stimulating agent (ESA) overall (OR 0.40, p = 0.0003) and as first line therapy (OR 0.51, p = 0.02). Thalidomide was also less commonly used in the POST patients (OR 0.34, P = 0.003). The use of hydroxyurea was similar between cohorts.
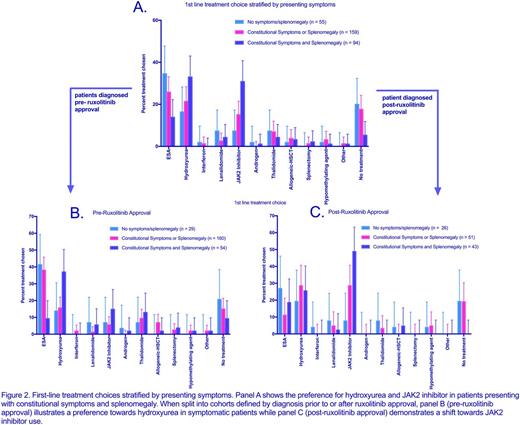
When stratified based on the presence of constitutional symptoms (CS) and/or splenomegaly (S), patients most commonly received an ESA, JAK-2 inhibitor, or hydroxyurea as frontline therapy (see figure 2). When both CS and S were present, patients more commonly received the latter two options. Patients presenting with CS and S were more likely to receive ruxolitinib as first line therapy in the POST cohort compared to the PRE (OR 5.5, p = 0.0004); however, the use of first line hydroxyurea was not significantly different between the PRE and POST cohorts (p = 0.28). In all, ten separate frontline treatments were used in the symptomatic PRE cohort while only five were utilized in POST patients.
In patients presenting without constitutional symptoms or splenomegaly, there was no difference in initial treatment strategy in the PRE and POST cohorts, and ruxolitinib was rarely utilized in this patient population.
No difference in overall survival (OS) was noted between the two cohorts.
Conclusion:After FDA-approval, ruxolitinib has emerged as the most common first-line treatment option for MF, specifically in patients with constitutional symptoms and splenomegaly, with decreased up-front use of thalidomide, ESAs and hydroxyurea. Patients presenting without constitutional symptoms or splenomegaly were no more likely to received ruxolitinib in the post-approval era, indicating a preference for alternative treatment options in this group. Longer follow up is needed to assess survival impact of frontline ruxolitinib use in our patient population.
Sweet:Karyopharm: Honoraria, Research Funding; Ariad: Consultancy, Speakers Bureau; Pfizer: Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Incyte Corporation: Research Funding. Komrokji:Novartis: Consultancy, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal