Abstract
Background: Tyrosine kinase inhibitors (TKIs) have dramatically improved outcomes in chronic myeloid leukemia (CML), but most patients either never achieve a complete molecular remission (CMR) or recur upon TKI discontinuation due to the persistence of leukemic stem cells. The kinetics of the initial TKI response appear predictive of long term outcomes as front-line imatinib patients who achieve a major molecular response (MMR) at 12 months have a 72% chance of attaining a CMR versus just 5% for those who do not (median time on Imatinib (IM) prior to CMR 66 months) (Branford. ClinCancer Res. 2007). Interestingly, patients who achieve and maintain a CMR for ≥2 years on single agent IM have been the subject of several TKI discontinuation trials demonstrating that 38-47% of such patients maintain treatment-free remissions (TFR) at 2 years (median time on IM prior to discontinuation of 50-70 months) (Mahon. Lancet Oncol. 2010 and Ross. Blood. 2013). Interferon-alpha (IFN) and K562/GM-CSF vaccines have been shown to enhance the frequency of deep molecular responses to TKIs (Preudhomme. N Engl J Med. 2010 and Smith. ClinCancer Res. 2010). We have shown that IFN targets LSCs, which may be the mechanism of improved IM responses (Angstreich. Br J Haematol. 2005). We now report initial outcomes for patients randomized between these treatments as a means of achieving TFR.
Methods: Patients with Ph+ CML in complete cytogenetic remission on first-line TKI treatment for ≥1 year were randomized to receive either K562/GM-CSF vaccination at 3 week intervals (vaccine) or daily interferon/GM-CSF (IFN/GM) in combination with their TKI. If CMR was achieved after a minimum of 6 months of adjuvant treatment, then all therapy was stopped, and monthly BCR-ABL PCR testing was performed to monitor for relapse. Based on the limits of detection of our assay, CMR is defined as at least MR4.3, which is IS <0.01. At disease recurrence, or if CMR was not achieved at 12 months of combination therapy, patients were allowed to cross-over. The treatment arms were defined as IFN alone (A), vaccine alone (B), IFN followed by vaccine (C), or vaccine followed by IFN (D).
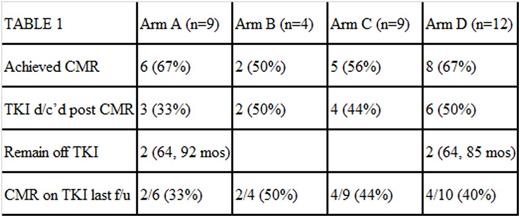
Results: 34 patients were enrolled with final treatment allocation shown in Table 1. Most patients were on IM (n=31), nilotinib (n=2) and dasatinib (n=1) with a median duration of TKI use at enrollment of 19 (range 12-80) months. Both arms of the trial were well-tolerated. The vaccine arm was notable for grade 1 and 2 injection site reactions in all patients. Predictably, the IFN arm produced grade 1 and 2 myalgias and flu-like symptoms in the majority of patients. Three patients had to discontinue treatment on the IFN arm due to grade 3 adverse events including a neurological event of unknown causality (1) and ALT elevation (2). Table 1 shows the responses by treatment arm with 62% of patients achieving CMR at a median of 28.6 (range 11-100) months of TKI therapy (compared to 66 months in IRIS). 15 patients (44%) discontinued TKI treatment per study plan after a median of 27.5 (range 19-56) months of TKI treatment (compared to median of 50-70 months of TKI in STIM/TWISTER), and 4 patients (27%) remain off therapy with TFRs of 64, 64, 85, and 92 months. Those with recurrence were off TKI for a median of 3 (range 1-5) months. Importantly, the long term responders who achieved CMR with adjuvant therapy all received IFN adjuvant, and all had relatively little prior TKI treatment (21, 27, 28, and 44 months) compared to published discontinuation studies. As expected in this group, all 4 had achieved a MMR at 12 months on TKI alone (p=0.05). What was unexpected was that 50% of patients treated with one of the IFN arms who had not achieved a MMR at 12 months on TKI alone went on to CMR after treatment on study compared to previous reports and just 15% of historical controls in our institutional database who achieved CMR on TKI alone (p=0.02)
Conclusions: Adjuvant therapies may improve the likelihood of achieving CMR and allow for discontinuation of TKI in CML patients with minimal TKI exposure. Even patients predicted to not achieve CMR based on a lack of MMR at 12 months did so at a higher rate than expected following IFN treatment. IFN likely played a role in long term TFR in 4 exceptional responders who successfully discontinued IM sooner than in previously reported discontinuation trials, possibly owing to IFNs effect on primitive CML progenitors.
Smith:Actinium Pharmaceuticals, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal