Abstract
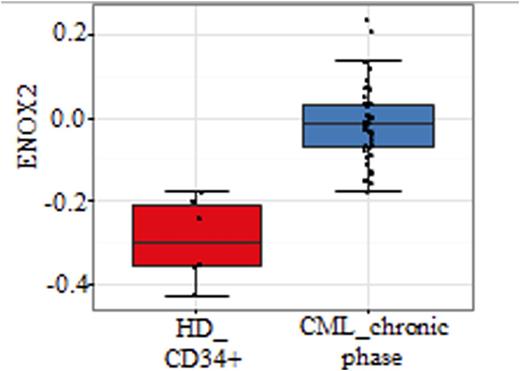
Ecto-nicotinamide Adenine Dinucleotide Oxidase 2 (ENOX2) or Tumor-Associated Nicotinamide Adenine Dinucleotide Oxidase (tNOX), plays a major role as a membrane hydroxyquinone oxidase catalyzing the conversion of reduced NADH to the oxidized NAD+ .Cellular NAD+/NADH ratio plays a major role in the regulation of several metabolic pathways such cell cycle and apoptosis. The membrane NAD+/NADH ratio has been shown to be involved membrane lipid and sphingomyelin metabolism. Several lines of experimental data indicate that ENOX2, as compared to ENOX1 isoform, is preferentially expressed in cancer cells and ENOX2 is also secreted in the serum of patients with cancer and representing a surrogate marker for cancer progression as well as a drug target. The expression of ENOX2 in CML has not been studied so far. To this end, we have analyzed the expression of ENOX2 by Western blots in 40 patients with CML at diagnosis and compared this expression to that of control cells from healthy donors (n= 30). ENOX2 expression was found to be highly increased ( x 6.6 fold) in primary leukemic cells and this was highly significant ( p= 0.0081). To determine if ENOX2 expression is related directly to BCR-ABL expression, we have analyzed the expression of ENOX2 in UT7 cell line and its BCR-ABL-expressing counterparts UT7-11. As compared to parental UT7, ENOX2 expression was highly increased in UT7 cells expressing either native or T315I-mutated BCR-ABL. This increase was also documented in DOX- inducible cell line BaF/p210 sin1.55 in which activation of BCR-ABL expression correlated with ENOX2 expression. The expression of ENOX2 in CML cells was a tyrosine kinase-dependent event as demonstrated by Western blot experiments showing that ENOX2 expression was reduced UT7/11 cell line treated with Imatinib mesylate (1 microM) for 6, 18 and 24 hours whereas there was no change in ENOX2 expression in similarly treated parental UT7 cell line. As ENOX2 is a protein secreted from tumor cells, we have analyzed the levels of ENOX2 in the plasma of CML patients at diagnosis as compared to controls. A series of 41 patients with CML as compared to plasma from 28 healthy donors were analyzed by ELISA. This analysis showed a highly significant increase of ENOX2 protein levels in the plasma of patients with CML (mean levels of 800 pg/ml in CML versus 500 pg/ml, Mann-Whitney U-Test, p < 0.0001). There was no correlation between ENOX2 levels and leukocyte numbers at diagnosis. In order to determine the potential clinical value of ENOX2 expression in different phases of the disease, ENOX2 expression in CML CD34+ cells was compared to healthy donor samples in microarray dataset GSE 4170. CML patients in chronic phase overexpressed ENOX2 in CD34+ cells as compared to control (two-sided test with Welch correction p-value=0.00054). Gene expression pattern matching correlating ENOX2 in CML CD34+ cells was determined in three phases of the disease. Pavlidis template matching algorithm used with ENOX2 as predictor allowed to discover 301 genes correlated with CML in chronic phase. Unsupervised principal component analysis performed with ENOX2 pattern matching gene expression profile allowed us to discriminate the 3 phases of CML in CD34+ cell compartment in a highly significant manner (p-value 6.75E-15). Functional enrichment performed with ENOX2 pattern matching on Gene Ontology Biological Process database revealed implication of different pathways of cell signaling such as: Rho GTPase, MAPKs, GPCR, RAS and NOTCH pathways, the latter being connected to stem cell biology. This analysis also showed implication of metabolic functions such as carbohydrate homeostasis. Other functionalities that could act on hematopoiesis have been also highlighted by this analysis such as proliferation, integrin-mediated signaling, and circadian rhythm. Finally genes related to angiogenesis have been also found to be implicated in ENOX2 signalling such as placental growth factor (PLGF) also EPHB3 which is a receptor tyrosine kinase implicated in cell migration. Overall these results suggest that ENOX2 pathway plays a major role in the pathogenesis of CML and represents, to the best of our knowledge, the first surrogate secreted tumor marker in CML. ENOX2 expression correlates with disease progression and experiments are underway to determine the use of ENOX2 as a drug target in CML and T315I-mutated CML stem cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal