Abstract
Background: Second-line salvage chemotherapy(CT) followed by high-dose (HD) CT and autologous stem cells reinfusion (ASCT) is standard treatment forR/R HL patients, although long-term cure can be achieved in only half of them, depending on risk factors. Chemosensitivity to salvage CT before ASCT, mainly represented by a negative PET scan, is highly predictive of a favorable outcome. The availability of new markers of prognosis, like the measurement of the serum chemokineTARC, could help identifying those patients who may require further treatment, i.e. new drugs like Brentuximab Vedotin or Nivolumab or Pembrolizumab, before ASCT, in order to achieve a durable remission.Therefore we planned to prospectively evaluate the prognostic role of serum TARC levels collected at different time points in cHL R/R patients.
Methods: Serum TARC levels were measured by commercially available ELISA test kits (R & D Systems, Minneapolis, USA) in 41 patients treated with IGEV (ifosfamide, gemcitabine, vinorelbine, prednisone) salvage CT, followed by myeloablative B(F)EAM (carmustine, (fotemustine), etoposide, cytosine arabinoside, melphalan) + ASCT at Istituto Nazionale Tumori of Milan, Italy, from January 2007 to December 2013. The 99th centile of TARC distribution in a group of 156 independent healthy subjects corresponding to 800 pg/mL, was considered as cut-off value discriminating between normal and abnormal TARC values. TARC evaluation was performed before starting salvage CT(T0), after the first IGEV cycle (T1) and before ASCT (T-preASCT). The Wilcoxon Mann Whitney test was used to analyze TARC as a function of patient and disease characteristics and PET results. Kaplan-Meyer curves and log-rank test were used to assess differences in PFS according to TARC levels. Cox model was used for multivariate analysis.
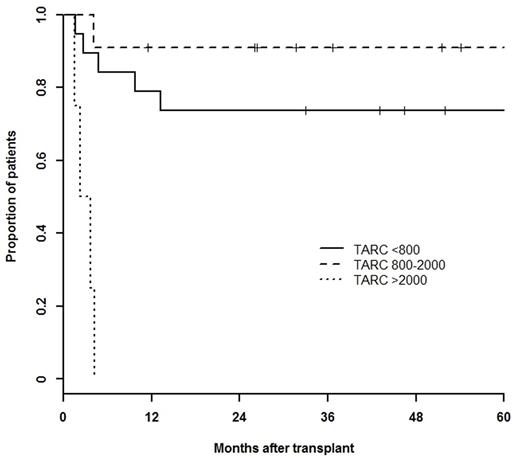
Results: Main patient characteristics at relapse/progression were as follows: males/females: 19/22, median age: 31 years (range,19-69), B symptoms: 34%, bulky disease: 27%, stage III/IV: 39%, extra nodal involvement: 34%, refractory vs relapsed < 12 months vs relapsed ≥ 12 months: 49% vs 34% vs 17%. Median (IQ range) T0, T1 and T-preASCT were: 1856 (8801-9983) pg/mL, 1148 (544-2532) pg/mL and 829 (454-1725) pg/mL, respectively. Patients with bulky disease had higher median T0 than their counterpart (3241 vs 1462 pg/mL; p=.016). Median T-preASCT was significantly higher in patients with refractory disease compared to relapse < or ≥ 12 months (1100 vs 595, vs 548 pg/mL, p=.027). A positive PET was recorded in 57.5% of patients after 2 IGEV cycles and in 32% before ASCT. Forty-one percent of patients needed ≥ 2 salvage CT before ASCT and 63% had ≥ 2 risk factors. At each time point, median TARC values were significantly higher in PET-2 positive patients compared to their counterpart (T0: 3238 vs 1310; T1: 1866 vs 624; T-preASCT: 908 vs 544; pg/mL). Median (IQ range)T-preASCT levels were significantly higher in patients with a positive PET before ASCT:1091 (596-10578) pg/mL compared to those with a negative one: 651 (447-964) pg/mL. After a median follow-up of 65 months, 5-year PFS and OS (95% CI) were 70 (57-88)% and 84 (72-98)%, respectively. In univariate analysis T-preASCT > 2000 pg/mL, PET-2, PET-preASCT, ≥ 2 risk factors and ≥ 2 salvage CT lines significantly affected PFS. In multivariate analysis only T-preASCT > 2000 pg/mL was significantly associated with a poor PFS (HR 6.65, CI95% 1.12-39.35, p=0.036) as shown in Figure 1.
Conclusions: Results of this single institution prospective study suggest that a cheap and easy to perform test, like serum TARC levels measurement before ASCT, may help to ameliorate the identification of those patients at risk of failing ASCT, for whom anticipated use of new active drugs, like anti-CD30 immunoconjugates and/or anti-PD1 blockers, should be explored, in order to improve the cure rate of ASCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal