Abstract
Introduction
While the role of cytarabine-based immunochemotherapy (ICT) and autologous stem cell transplantation (ASCT) in untreated younger patients with MCL is well established, the utility of 'more intense' approaches in older patients is uncertain. Despite a median age at presentation of 60 years or more there are few therapeutic trials focussing on older patients. With the development of targeted agents, the need to define the optimal ICT strategy for comparison is pressing.
Methods
The primary aim of this study was to compare the safety and efficacy of first line ICT based therapy in patients with MCL aged over 60 years. Treatment and outcome data were collected for patients treated between 2000-2015 across a combined health-care network servicing the south-eastern, south-western and central corridor of the Australian state of Victoria (catchment area >2 million patients encompassing four major academic tertiary referral centers). Eligible patients had MCL diagnosed according to WHO 2008 criteria, confirmed by either fluorescence in situ hybridization for t(11;14) translocation or immunohistochemistry for cyclin D1 expression. Only cases with adequate information, including baseline characteristics, treatment regimens and outcome, were included. Overall survival (OS) and progression free survival (PFS) were modelled using Cox regression. For ICT comparison, patients undergoing ASCT were censored at the time of transplant. Differences in hospitalization and transfusion were analyzed using the Mann-Whitney U test.
Results
52 patients met inclusion criteria with a median age of 69 (range 60-91; M/F >3:1), of which 23 patients were >70 years. Most patients had advanced disease on staging, >1 extra-nodal site and an intermediate-high MIPI score. All received rituximab with initial therapy. Treatment regimens included: R-CHOP-like (31), alternating R-CHOP/R-DHAC (10), R-HyperCVAD/R-MA (7) and other (4). Eleven patients underwent ASCT. The median follow up for surviving patients was 40 months.
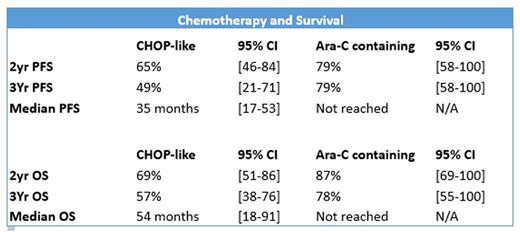
Type of chemotherapy and up-front ASCT were the only variables influencing PFS and OS. Cytarabine-based ICT was associated with an improved median PFS (not reached vs 35 months, HR 0.27 [0.08-0.93], p=0.018) and OS (not reached vs 54 months, HR 0.28 [0.08-0.94], p=0.039) compared to R-CHOP-like regimens. Patients undergoing ASCT were younger (mostly < 70 years) and demonstrated improved median OS (not reached vs 46 months, HR 0.124 [0.017-0.921], p=0.041). No statistically significant difference in efficacy between R-HyperCVAD/R-MA and R-CHOP/R-DHAC could be determined due to low numbers. Both group of patients receiving cytarabine demonstrated durable response over time. However, those receiving R-HyperCVAD/R-MA experienced greater toxicity with increased hospitalisation: median 54 [31-72] v 20 [6-77] days, p=0.023 and transfusion requirements: median 13 [7-24] v 2 [0-5] red cell units, p=0.001; median 6 [2-15] v. 0 [0-1] platelet pools, p=0.001.
Conclusion
In older patients with previously untreated MCL, cytarabine-based ICT resulted in a significant improvement in OS, with a 72% reduction in the risk of death relative to CHOP-like therapy. Cytarabine-based ICT is highly efficacious and well tolerated and should serve as a benchmark with which to compare targeted therapies. ASCT should also be considered in selected older patients.
Quach:Amgen: Membership on an entity's Board of Directors or advisory committees; Janssen Cilag: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Opat:Roche: Consultancy, Honoraria, Other: Provision of subsidised drugs, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal