Abstract
Introduction: Entospletinib (GS-9973) is an orally bioavailable, selective inhibitor of spleen tyrosine kinase (Syk). Syk is a mediator of B-cell receptor signaling in normal and transformed B-cells. Targeting the B-cell receptor (BCR)-signaling pathway has been the focus in many types of B-cell related hematological malignancies including mantle cell lymphoma (MCL).
Methods: This reports the MCL cohort in a phase 2 trial that more broadly evaluated the efficacy and safety of entospletinib (800 mg BID) in patients with relapsed and refractory hematological malignancies (NCT01799889). Tumor response was assessed per Cheson 2007 criteria with imaging planned at weeks 8, 16, 24, and then every 12 weeks. The primary endpoint was PFS at week 16. All efficacy data were assessed by an Independent Review Committee.
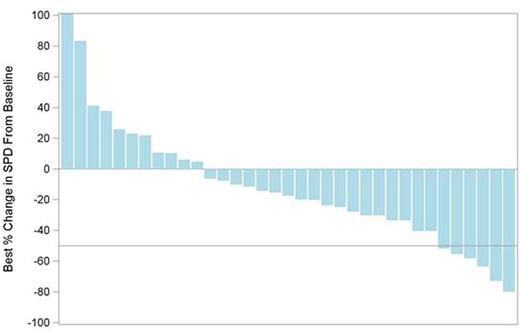
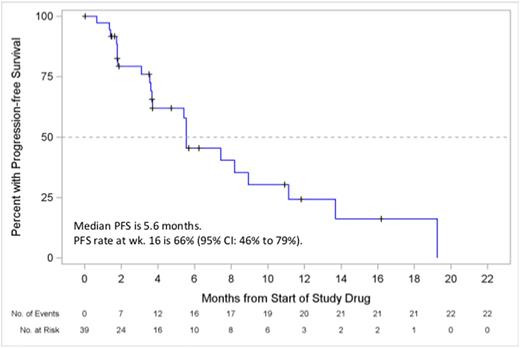
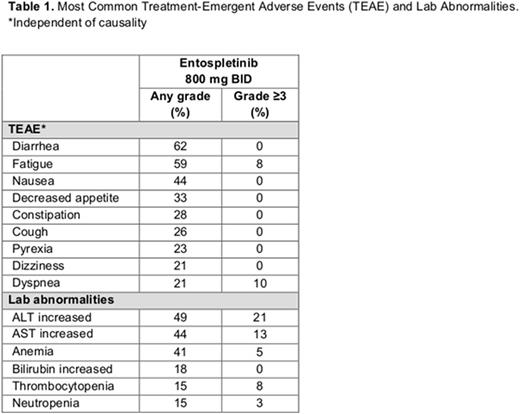
Results: A cohort of 39 patients with MCL was enrolled. The median age was 72 years (range: 49-92), 64% were male, and a median number of prior regimens were 2 (range: 1-6). Prior therapy included anti-CD20 antibodies (95%), alkylating agents (95%; bendamustine 44%), and anthracyclines (77%). Three patients (8%) have received ibrutinib either as investigational drug (n = 1) or as an approved drug (n = 2), and 1 patient (3%) received idelalisib. Median duration of treatment was 21 weeks (range: 1-87), with 1 patient continuing on treatment. Thirty-five (90%) patients were evaluable for tumor response. Four patients (10%) discontinued prior to initial tumor assessment: death (n = 1), disease progression (n = 2) and investigator's discretion (n = 1). The most common TEAEs (any grade/≥gr 3, independent of causality) and common lab abnormalities are summarized in Table 1. There were 4 TEAEs that led to study drug discontinuation (all n = 1): cardiac arrest, pruritus, pyrexia, and maculopapular rash. Six deaths were reported, none of which were related to study drug. The ORR was 15% (90% CI: 6.9%, 28.1%), with 6 (15%) patients achieving a PR and 23 (59%) patients maintaining a stable disease. The PFS rate at week 16 was 66% (95% CI: 46%, 79%). Median PFS was 5.6 months (95% CI: 3.6 months, 8.9 months). These results are based on data analysis of June 28, 2016.
Conclusion: Entospletinib was well tolerated and demonstrated modest activity in patients with relapsed or refractory MCL. Further development of entospletinib in MCL will focus on the development of combination therapy.
Sharman:Gilead Sciences, Inc.: Honoraria, Research Funding. Kolibaba:Amgen: Research Funding; Celgene: Research Funding; Cell Therapeutics: Research Funding; Genentech: Research Funding; GSK: Research Funding; Janssen: Research Funding; Acerta: Research Funding; Gilead: Consultancy, Research Funding; Novartis: Research Funding; Pharmcyclics: Research Funding; Seattle Genetics: Research Funding; TG Therapeutics: Honoraria, Research Funding. Shustov:Seattle Genetics: Research Funding; BMS: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; SPECTRUM: Consultancy, Research Funding; Novartis: Research Funding. Nay:Gilead Sciences, Inc.: Honoraria; Janssen: Honoraria, Other: Advisory board; Celgene: Honoraria, Other: Advisory board; Amgen: Other: Advisory board; Lyndbeck: Honoraria; BMS: Honoraria. Zhang:Gilead Sciences: Employment, Equity Ownership. Shi:Gilead Sciences, Inc.: Employment, Equity Ownership. Forero:University of Alabama at Birmingham: Research Funding. Assouline:BMS: Speakers Bureau; Lundbeck: Consultancy; Janssen: Consultancy, Speakers Bureau; Pfizer: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal